Acute non-ST-segment elevation myocardial infarction (NSTEMI) initial approach includes antithrombotic treatment. The duration, moment of treatment initiation and drug choice will depend on procedure-related and patient-related factors, always taking into account both ischemic and haemorrhagic risk1.
Standard treatment for these patients consists of initial dual antiplatelet therapy (DAPT), with a cyclooxygenase inhibitor and an adenosine diphosphate (ADP) receptor inhibitor, followed by single antiplatelet therapy.
Within the ADP receptor inhibitors, two groups can be differentiated according to their structure: thienopyridines (clopidogrel and prasugrel) and non-thienopyridines (ticagrelor). Ticagrelor and clopidogrel are considered potent P2Y12-inhibitors, and are the drugs of choice in patients with NSTEMI1.
Thrombotic thrombocytopenic purpura (TTP) is a severe, multisystemic and rare complication with an annual incidence of 6 cases per million inhabitants and is often related to a congenital or acquired deficiency of the ADAMTS13 enzyme, connected to the degradation of von Willebrand factor (vWF)2. Cases of acquired TTP have been reported in association with drug treatments (such as ticlopidine and clopidogrel), infections, pregnancy, cancer, etc3.
We present the first reported case of an exacerbation of TTP in a patient with DAPT after a NSTEMI. The initial episode occurred during treatment with ticagrelor, and the exacerbation after restarting DAPT with clopidogrel.
Case reportAn obese, former smoker male patient in his 60s, with no medical history of interest was admitted to the hospital due to a NSTEMI, coronary artery disease of the anterior descending coronary artery revascularized with a stent and dilatation of the aortic root. He was diagnosed with arterial hypertension, type 2 diabetes and dyslipidemia. He was discharged without complications after three days with the following medication: omeprazole 20 mg oral daily, acetylsalicylic acid (ASA) 100 mg oral daily, ticagrelor 90 mg oral twice a day, bisoprolol 2,5 mg oral twice a day, atorvastatin 80 mg oral daily, empaglifozin/metformin 5 mg–1000 mg oral twice a day, enalapril/lercanidipine 20 mg–10 mg oral daily, nitroglycerin if chest pain and deflazacort 75 mg in a descending pattern.
One week after discharge, the patient contacted the emergency services due to paresthesia in the left upper limb, left hemiparesis and aphasia, for which he was transferred to the intensive care unit (ICU).
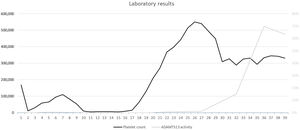
A cranial computed tomography (CT) scan was performed, showing no ischemic or hemorrhagic findings; as well as blood test showing severe thrombopenia (11,000 platelets/μL), hemoglobin 12.4 g/dL, lactate dehydrogenase 1761 U/L, ADAMTS13 activity <0.2%.(Fig. 1) These results led to the diagnosis of TTP with neurological involvement.
This complication was suspected to be related to the administration of ticagrelor, which led to its suspension on admission and continuation of antiplatelet therapy only with ASA. Daily plasma exchange therapy and IV-corticotherapy were started.
Three days later, after a slight increase in platelet counts (65,000 platelets/μL) (Fig. 1), he was transferred to the hospital ward and DAPT was restarted, replacing ticagrelor with clopidogrel. Five days after the addition of clopidogrel, the patient's neurological condition worsened, as platelet count decreased to 15,000 platelets/μL. Clopidogrel was suspended, the patient readmitted to the ICU, treatment with IV corticotherapy and plasma exchange therapy was continued and treatment with IV rituximab was initiated. The platelet count reached its minimum, 5,000 platelets/μL, six days after the introduction of clopidogrel.
When refractoriness to the established treatment was observed, concurrent treatment with caplacizumab was started intravenously and continued subcutaneously for a total of 30 days, until a favorable evolution of the patient was observed, with recovery of platelet counts and no hemorrhagic or thrombotic events, which allowed the suspension of plasma exchange therapy after 16 sessions, rituximab treatment after three weekly doses and IV-corticotherapy after 53 days.
After seven weeks of hospitalization the patient was stable, with progressive recovery of neurological alterations, with platelet counts of 331,000 platelets/μL and ADAMTS13 activity of 63.5% (Figure 1). He was discharged and chronic medication was reintroduced. During the follow up period, no other adverse events (AE) or complications were observed and ADAMTS13 activity increased up to 101% ten weeks after discharge.
DiscussionClinical practice guidelines recommend TDAP after NSTEMI regardless of the performance or not of an invasive procedure. TDAP has a beneficial effect in reducing stent-related thrombotic events and acute myocardial infarction. Ischemic and hemorrhagic complications have an important influence on the evolution of patients with NSTEMI and on the overall risk of death4.
TTP is a life-threatening disease, with a mortality rate of 10–20% despite adequate first-line treatment5. Classically, the acute phase of TTP was characterized by the onset of: fever, thrombocytopenia, microangiopathic hemolytic anemia, neurological disorders and renal failure, however it has been observed that this pentad occurs in less than 10% of patients. The definition of TTP has recently been completed by the presence of a severe deficiency of ADAMTS13 enzyme activity, specific for TTP3,4.
Ticagrelor is the most recently marketed P2Y12-inhibitor; it differs from clopidogrel and prasugrel in its structure, since it is a cyclopentyl-triazolopyrimidine; in its mechanism of action, which is reversible; and in its pharmacodynamics, not requiring metabolization after absorption for platelet inhibition1.
In the World Health Organization pharmacovigilance database (VigiBase®), 15 cases of TTP have been reported with ticagrelor and 447 with clopidogrel6. The DAPT and NSTEMI management guidelines do not mention TTP as a possible AE related to P2Y12-inhibitors or its management4.
There is some controversy about the approach in patients who develop TTP as an AE to P2Y12-inhibitor therapy7. A case of TTP has been reported in a patient treated with ASA who had suffered a previous TTP on clopidogrel8, however, there is no reported case of TTP with a P2Y12-inhibitor after a previous TTP. Some authors recommend restarting DAPT after recovery from the initial thrombopenia7. However, in our patient, an exacerbation of the TTP episode was observed after restarting DAPT.
Although the mechanism by which P2Y12-inhibitors may be involved in the occurrence of acute TTP is unknown, there are authors that find those related to ticlopidine immune-mediated, and the remainder associated with endothelium damage or stimulation, leading to vWF secretion. In this case, the low ADAMTS13 activity suggests an immune-mediated mechanism, which differs from other authors’ approach9, revealing that ticagrelor induced TTP could be mediated by several mechanisms and not only endothelium damage. This reflects the importance of having more research on this topic.
According to the Naranjo causality algorithm, there was a probable association for both drugs with the AE, with a score of five with ticagrelor and an score of six with clopidogrel, ruling out their reinitiation10.
In conclusion, we describe the development of an exacerbation of a previous TTP episode in a patient with P2Y12-inhibitors. The patient developed a TTP with ticagrelor, a reaction not well described in the literature, and subsequently, after restarting TAPD, an exacerbation of the TTP with clopidogrel, a better known association.
AuthorshipAll authors meet the ICMJE authorship criteria. L. Macía-Rivas was responsible of conceiving the idea and contributed in every process. I. Maray was part of the idea development, made a literature review, contributed with scientific writing and final approvement. C. L. Fernández-Laguna, C. Álvarez-Asteinza and A. Lozano-Blázquez helped with revision of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical considerationsInformed consent was signed by the patient included in this case report.