In 1992 Medicus Mundi Mediterrània and the Sahrawi Arab Democratic Republic spearheaded a cooperation project to set up a small drug compounding laboratory in the Sahrawi refugee camps located in the desert town of Rabouni (Algeria's Tindouf province). The goal of the project was to build a drug compounding facility, create a training center for local staff, and ensure local production of medicines in cases of inadequate supply.
With the help of external experts and of multiple public and private organizations and institutions, a small compounding laboratory was established with separate work areas, similar to the lines of the laboratories found in Spanish hospitals. The laboratory now has a stable team of 10-12 pharmacy technicians led by a pharmacist. In the last 25 years, 40 Sahrawis have been trained through internships in hospital pharmacy departments abroad, visits to drug compounding labs in other countries, tutoring, and ongoing training programs. Products that can be compounded in the laboratory include capsules, oral solutions, creams and ointments, topical solutions, eye drops, and sterile irrigation and lavage solutions. Over fifty different formulations of varying degrees of complexity have been prepared. One of the most significant challenges, given the political situation and the geographical environment, has been the procurement and transportation of the active ingredients and the packaging materials/equipment required for the compounding process. After 25 years of continuous work, this project is now a reality thanks to the effort and direct involvement of the Sahrawi people. The experience gained in the last few years has shown the importance of coordinating drug preparation with local prescribing physicians, as well as having quality standards and facilities with homologated low complexity basic equipment that allows, in other areas that is required, the replication of this model.
En 1992, Medicus Mundi Mediterrania y la República Árabe Saharaui Democrática iniciaron un proyecto de cooperación para el establecimiento de un pequeño laboratorio de producción de medicamentos en los campamentos de refugiados del pueblo saharaui, situado en pleno desierto del Sahara, concretamente en Rabuni, Tindouf (Argelia). Los objetivos del proyecto fueron establecer una estructura e instalaciones que permitieran la elaboración de medicamentos, crear un centro de formación de personal local y facilitar cierta capacidad de producción en situaciones de crisis o emergencia ante la falta de medicamentos.
Mediante el asesoramiento externo y la ayuda de múltiples organizaciones, instituciones y entidades privadas, se construyó un pequeño laboratorio de características similares al que teníamos en los hospitales españoles, con áreas diferenciadas de trabajo. El laboratorio dispone de un equipo humano estable de 10-12 técnicos superiores de farmacia dirigidos por un farmacéutico. A lo largo de estos 25 años se han formado y capacitado 40 saharauis, a través de estancias externas en servicios de farmacia, visitas a laboratorios de producción de otros países, tutorización de farmacéuticos y cursos de formación continuada. Se han incluido varias líneas de producción de medicamentos como: cápsulas, soluciones orales, cremas y pomadas, soluciones tópicas, colirios, soluciones de irrigación y lavado estériles. Se han elaborado más de 50 formulaciones distintas, todas ellas de diferente grado de complejidad. Una de las limitaciones más relevantes, por la situación y entorno, fue y sigue siendo la adquisición y envío de materia prima, material de acondicionamiento y/o equipos, necesarios en cualquier proceso de elaboración. Después de estos 25 años de trabajo continuado se evidencia la realidad de este proyecto, fruto del esfuerzo e implicación del pueblo saharaui. La experiencia adquirida en estos años plantea la extrema necesidad de coordinar las actividades de elaboración con los equipos médicos y/o prescriptores de la zona, así como disponer de estándares de calidad e instalaciones con equipos básicos homologados y de baja complejidad, que faciliten la replicación del modelo en distintos ámbitos y/o zonas de actuación.
In 1992, at the request of the Ministry of Public Health of the Sahrawi Arab Democratic Republic (RASD), a delegation of pharmacists from Medicus Mundi Mediterrània (MMMed) visited the Sahrawi refugee camps. These camps, which were create back in 1975, near the city of Tindouf (Algeria), host an estimated population of 150,000 people, distributed into five settlements (or wilayas) located around 18 miles from each other. Refugees live in a state of perpetual temporariness and insecurity and are totally dependent on external aid (Figure 1).
Organizationally, the camps’ healthcare services follow a hierarchical structure akin to that of the Spanish health system. Accordingly, the Ministry of Public Health is organized into central directorates devoted to prevention, medical assistance, medicines and equipment, personnel, logistics and veterinary services1. The system comprises a national hospital, a mixed-care hospital, and a regional hospital in each wilaya. At local level, there are 26 dispensaries located in the different districts (dairas) into which each wilaya is divided.
The Medicines Directorate is responsible for the Central Pharmacy, which receives all the medicines coming from abroad and then distributes them to the different wilayas. Although there is an official list of essential medicines, based on the one published by the WHO, the scarcity of pharmaceutical resources makes it impossible to meet the needs of all the refugees.
The compounding laboratory examined in this article is frame whiting the Central Pharmacy Directorate and the manufactured medicines are part of the medicines’ circuit established for the camps.
Another basic public service available in the camps is the educational program run by the Ministry of Education, which offers basic education to all Sahrawi children. Once they complete their basic compulsory education, youths are offer the opportunity to pursue higher education and/or university degree in one of the countries that support the RASD.
In this respect, it should be mentioned that a local nursing school was inaugurated in 1995, which has since then trained nurses and midwives who, after obtaining their official degree, typically join one of the different healthcare facilities in the area to render their indispensable and highly appreciated services.
However, the local healthcare staff is not enough to cater for the needs of the population. As a result, health care multidisciplinary teams (physicians, surgeons, pharmacists, nurses, equipment maintenance staff, etc.) from other countries, travel regularly to the area to address the needs that cannot be met by the local staff.
When a MMMed team visited the RASD refugee camps for the first time in 1992, they noticed that the local staff was preparing an antiseptic in a small dispensary. That gave them the idea of setting up a small laboratory where drugs could be compounded at a larger scale, thereby helping address some of the most urgent healthcare needs of the local population. The laboratory would have to be a highly functional facility, adapted to the situation of the camps and to the environment around it, and following MMMed's philosophy, it would have to support the Ministry of Health in strengthening the local healthcare structure and promote the professional development of local healthcare providers who did not have many possibilities to find a job in their field of expertise.
Once the Sahrawi authorities gave their approval and the idea was presented to different aid institutions, organizations and agencies, both at a local and at the Spanish level, a decision was made to submit the project to different calls for funding by different public aid agencies. Thanks to the financing and support from different entities and organizations, the project saw the light and has continued to this day as a development project set in a refugee camp where interventions are based almost exclusively on humanitarian aid. The project had four main goals:
- •
To construct a drug compounding facility endowed with the necessary equipment.
- •
To establish a training center aimed at instructing local personnel in the techniques used in drug compounding and in the management of a compounding laboratory.
- •
To promote the capacity to prepare drugs against the backdrop of critical or emergency situations, and of the dearth of some active ingredients.
- •
To lay the foundations for the future development of a national drug compounding laboratory when the current situation improves and Sahrawis are allowed to move back to Western Sahara.
The drug compounding laboratory is located in the hospital area at Rabouni, which is the administrative capital of the Sahrawi refugee camps, about 16 miles from the Algerian city of Tindouf.
One of the most important tasks was the design and planning of the structure of the laboratory, which would be divided into different sections and areas in order to ensure efficient operation. Once the construction work was completed, the equipment required for the proper functioning of the different production lines was acquired. At the same time, accredited active ingredient and packaging material vendors were identified that could provide the inputs required for the different preparations to be produced2,3.
One of the main hurdles to be overcome was the sourcing of pharmaceutical-quality water in an area characterized by extreme weather conditions where ground water is unfit for drinking and of great hardness given its high salt content. After testing different methods, techniques and procedures, it was decided to install a decalcification unit, together with a particle filtering system and a Millipore Elix 3® water purification system.
A theoretical/practical training program was progressively introduced, led by the Sahrawi pharmacist in charge of the laboratory to provide the staff with instruction in the proper management of the laboratory, in the compounding of preparations, and in the maintenance of the facility and the equipment. The program was give to 10 staff members, most of them with a background in pharmacy, chemistry, microbiology, etc., which composed the first team to operate the laboratory.
A digital platform was recently introduced to record, store, access and manage the information and documents belonging to the laboratory.
The project benefited from the guidance of the Servei de Desenvolupament del Medicament (SDM) of the Department of Galenic Pharmacy of the University of Barcelona, the Dr. Carreras pharmacy in Barcelona, the Grifols laboratory, the Pharmacy Department of Barcelona's Hospital Clínic, and a series of specialists who provided their input when requested to do so. The most comprehensive literature on the subject was also reviewed4-8.
ResultsStructure and equipmentEver since its inauguration in 1996 (Figure 2), the drug compounding laboratory has had an office with IT equipment, a meeting room with audiovisual equipment, a locker room with its own toilet, three small store-rooms, a water production and sterilization area with a Millipore Elix 3® unit and two autoclaves, a poupinel oven, a water bath, a blender and shakers, and a small area for analyses and quality control containing a spectrophotometer, a pH meter, and a boiling point apparatus. In 2015, two clean rooms were installed, one of them with a horizontal laminar air flow cabinet and another one with two encapsulation devices.
Human resourcesThe laboratory's permanent staff comprises a mean of 10-12 people. In the last few years, about 40 Sahrawis have been trained in drug compounding, quality control, basic equipment maintenance, and management. Three of the staff members have been there from the start. The training program has consisted in five internships at the Pharmaceutical Technology Laboratory of the Hospital Clinic, one internship at the Electromedicine Department of the same hospital, two internships at the SDM of the School of Pharmacy of the University of Barcelona, and five study visits to the pharmaceutical technology laboratories of other hospitals and to community pharmacies specializing in drug compounding, as well as ongoing training by fellow laboratory staff, and instructional sessions (twice a year) by external voluntary workers.
The main added value of the laboratory staff is that every staff member acts as a trainer of new personnel, instructing them on concepts, norms, and work procedures.
ProductionThe division of the laboratory into different work areas and the availability of new equipment have allowed the implementation of several compounding lines:
MedicinesThe following medicines and pharmaceutical formulations have been prepared:
Capsules: amoxicillin 500 mg, metronidazole 250 mg; cotrimoxazole 400/80 mg; erythromycin 500 mg; doxycycline 100 mg; rifampicin 300 mg; folic acid 1 mg; omeprazole 20 mg; paracetamol 500 mg; ibuprofen 400 mg; loperamide 2 mg; metoclopramide 10 mg; prednisone 5 & 10 mg; ivermectin 3 & 6 mg; metformin 850 mg.
Oral solutions: amoxicillin suspension 125 mg/5 mL; carbocysteine syrup 5%; milk of magnesia; ibuprofen suspension 120 mg/5 mL; simple syrup; nystatin suspension 100,000 IU; ferrous sulphate syrup 8 mg/mL; dextromethorphan syrup 15 mg/mL.
Ear drops: boricated alcohol 5%; ciprofloxacin eye drops 3 mg/mL; gentamycin/dexamethasone eye drops 3 mg/mL. Eye drops: timolol eye drops 5 mg/mL; latanoprost eye drops 50 µg/mL; gentamycin eye drops 3 mg/mL.
Creams, ointments, and gels: camphor cream; diclofenac sodium cream 1%; emollient cream base; gentamycin cream 0.1%; hydrocortisone cream 1%; miconazole emulsion 2%; neomycin/bacitracin cream 5 mg/500 IUs; silver nitrate solution 2%; Lassar's cream; ultrasound gel; silver sulfadiazine cream 1%; sulfamethoxazole cream 1%; salicylic vaseline cream 5 a 20 %; permethrin solution 5%.
Antiseptic solutions: peroxide 3%; eosin 2% aqueous solution; potassium permanganate 1/10,000; povidone-iodine aqueous solution 10%; povidone-iodine soap solution 7.5%.
Other: sterile double distilled water; anti-itching powder; oral lowsodium rehydration powder; sodium chloride sterile irrigating solution 0.9% 3-liter bag; glycine sterile irrigating solution 1.5% 3-liter bag.
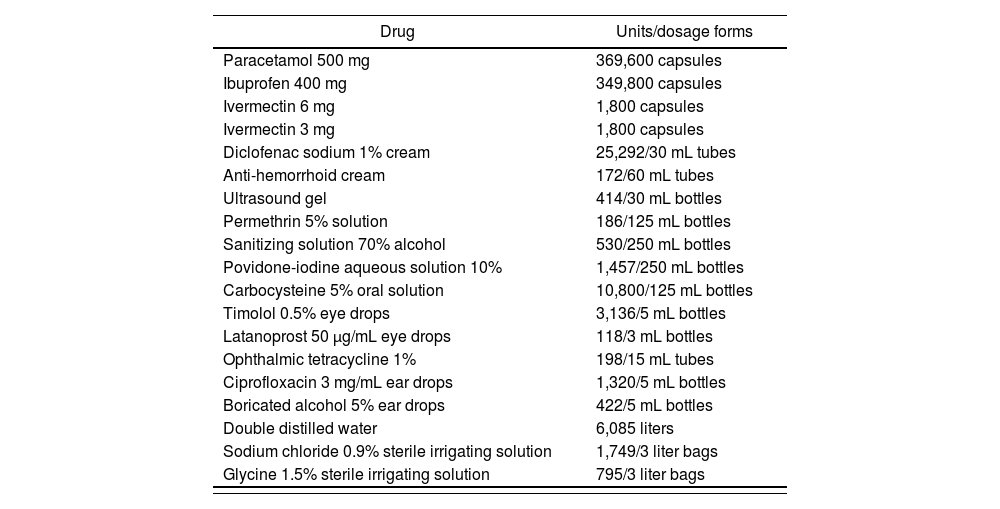
Table 1 contains manufacturing list of the most representative drugs compounded in the last three years (2018-2020).
Production of medicines at the SADR compounding facility in the last three years (2018-2020).
| Drug | Units/dosage forms |
|---|---|
| Paracetamol 500 mg | 369,600 capsules |
| Ibuprofen 400 mg | 349,800 capsules |
| Ivermectin 6 mg | 1,800 capsules |
| Ivermectin 3 mg | 1,800 capsules |
| Diclofenac sodium 1% cream | 25,292/30 mL tubes |
| Anti-hemorrhoid cream | 172/60 mL tubes |
| Ultrasound gel | 414/30 mL bottles |
| Permethrin 5% solution | 186/125 mL bottles |
| Sanitizing solution 70% alcohol | 530/250 mL bottles |
| Povidone-iodine aqueous solution 10% | 1,457/250 mL bottles |
| Carbocysteine 5% oral solution | 10,800/125 mL bottles |
| Timolol 0.5% eye drops | 3,136/5 mL bottles |
| Latanoprost 50 μg/mL eye drops | 118/3 mL bottles |
| Ophthalmic tetracycline 1% | 198/15 mL tubes |
| Ciprofloxacin 3 mg/mL ear drops | 1,320/5 mL bottles |
| Boricated alcohol 5% ear drops | 422/5 mL bottles |
| Double distilled water | 6,085 liters |
| Sodium chloride 0.9% sterile irrigating solution | 1,749/3 liter bags |
| Glycine 1.5% sterile irrigating solution | 795/3 liter bags |
At present, the range of medicines compounded has been cut down in order to free up capacity for the production of the drugs in highest demand because of not being included on the national medicines list, because imports of those drugs always fall short of demand, or because they are targeted at specific conditions. Moreover, compounding of certain penicillinbased drugs has been suspended either because of the pharmacological policies of the camps or because of the lack of the infrastructures required to produce them.
Quality controlThe raw materials used in the laboratory come from specific accredited manufacturers and each consignment of active ingredients, excipients or other ancillary materials must come with the required certificate of analysis2,3. In addition, protocols have been established for the control and calibration of precision equipment such as scales, pH-meters, volumetric pumps, spectrophotometers, filtration systems, and thermometers. Moreover, the water used in the laboratory is subjected to regular physical-chemical and microbiological analyses. Proper functioning of the Millipore® water purification system is checked periodically and proper sterilization of autoclaves is monitored using spores and colorimetric test strips.
Control of the compounding process includes weight control, double checking of the riskier tasks such as identification of active ingredients and/ or dilutions, visual control of the presence of particles, microbiological control of eye drops, etc.
Microbiological, environmental, and surface quality controls are carried out using kits for aerobic microorganisms, enterobacteria, fungi and yeasts, and coliform bacteria.
As regards the management of waste, the building that houses the laboratory is equipped with specific areas from which the waste is collected and subsequently processed, following Algerian law.
A series of failure mode and risk analyses have been conducted recently to sensitize the staff about the importance of preventing avoidable errors. A specific methodology has also been introduced to this effect5.
Documentary managementIn 2020, a digital storage platform was implemented containing information on the laboratory and its operation, which includes:
- •
Store-room management. Entries and exits, and stocks of raw materials, packaging materials, consumables, and personal protective equipment.
- •
Drugs compounded at each production line.
- •
Medicines manufacturing technical sheets.
- •
Documents of standard operating procedures.
- •
Training materials and video-tutorials of the different stages of the compounding process.
- •
Bibliographic archive with reference documents on compounding and compounding best practices, equipment catalogs, etc.
Over the last 25 years an infrastructure has been built and equipped to allow compounding of a considerable range of pharmaceutical formulations despite the difficulties of operating in a refugee camp and the extreme climate conditions prevailing in the desert. Those conditions require more time and resources to be invested in the maintenance, repair, and replacement of equipment than in countries like Spain.
The laboratory is entirely dependent on external aid coming from public and private donors, so every year it is necessary to look for resources needed, since any disruption in the inflow of funds potentially affecting the stability of the drug compounding process.
Under no circumstances is the objective of the laboratory to cater for 100% of the medication needs of the population. As mentioned above, the purpose was -from the onset- to provide the camp with a facility capable of producing the most frequently used medicines, an educational center to train the local staff in the compounding process, and the possibility to mitigate drug shortages, provided that enough raw materials were available.
Indeed, availability of raw materials is an important limiting factor as, given the dearth of such ingredients within the camps and in the Algerian market, it is necessary to import them from abroad, mostly from Spain.
Although it is true that the starting point goal was to produce a wide range of drugs, after a few years of operation it became clear that it would be more beneficial to limit the number of drugs produced so that healthcare authorities were clear about what drugs, and in what quantities, could be produced by the laboratory so that they could make their estimations.
A collaboration network with physicians from different specialties such as dermatology, ophthalmology and urology has been established in the last few years with a view to more accurately tailoring treatments to the patients’ needs. Specifically, collaboration with ophthalmologists has allowed preparation of eye drops, mainly for treating glaucoma and other eye diseases. Similarly, collaboration with urologists has resulted in the preparation of sterile bladder irrigation solutions in 3-liter glycine and sodium chloride bags, which until recently had to be imported from abroad.
This collaborations are highly beneficial for patients as local production of medicines allows a more efficient balance between supply and demand. Collaboration between prescribing physicians and the compounding laboratory should be promoted so as to optimize the scarce resources available and improve health outcomes.
The laboratory also collaborates with the Veterinary Department of the camps, compounding formulations to address livestock-specific diseases.
The precarious situation of the people living in the camps typically results in a high turnover of laboratory staff. Although they do receive a low stipend for their work, most staff members are obliged to look for other income sources to be able to support their families. This means that over the years approximately 40 people have been part of the laboratory team.
As in other working environments, training is variable. Although it is usually provided onsite by external volunteers and/or aid workers in the course of the two annual trips, it may also be imparted offsite, during the different internships the staff benefits from in hospitals abroad. However, the COVID-19 pandemic has made it necessary to implement online training sessions. The Spanish Society of Hospital Pharmacists (SEFH) signed an agreement with the laboratory to provide courses for pharmacy technicians. Also, IT systems have been installed to permit online meetings when internet connections are stable enough.
Availability of a digital platform allows for training to continue remotely, offering the laboratory staff instructional programs and video-tutorials on new compounding methods or procedures. This facilitates access to training as the staff can fit the training sessions around their schedule. The platform also allows an online communication channel to be established with volunteers and/or aid workers who can access information about the situation of the laboratory in real time.
The platform could also become an efficient tool for applying a quality assurance program to each stage of the compounding procedure. This would require for a new function to be introduced, which would provide users with systematic reminders of the different checks, calibrations, and follow-ups that need to be performed of the different processes and procedures.
One of the limitations of this cooperation project is its strong reliance on external assistance. If the project had not been conducted in a refugee camp, securing adequate funding would have been a significant challenge. Simulated sales could constitute a source of income that could enable the laboratory to buy the medications and equipment needed and be less dependent on external aid.
After our long-standing experience in the field of humanitarian aid, several reflections come to mind:
The first one has to do specifically with the compounding of drugs. Laboratories such as the one discussed in this article are small-scale facilities akin to those in the pharmacy departments of Spanish hospitals.
We believe that in order to ensure continuing implementation of best compounding practices, save design time, and apply standard operating procedures, validated, fit-for-purpose and adapted mobile “container-type” compounding units could be introduced. These units, built in a foreign country, could be sent to low-income countries where they could be entrusted to well-training staff who would count on all the necessary equipment to compound the drugs needed by the population. This has been called a “turnkey” solution as it allows immediate implementation of different production lines.
The second reflection is addressed to the profession of hospital pharmacists. Both SEFH as a scientific society and several SEFH members, either individually or through different NGOs such as Fundación el Alto9, have collaborated with or given their support to different cooperation projects across the different disciplines of hospital pharmacy. It would be advisable to join forces and leverage all possible synergies to bring together a group of like-minded professionals to debate, share ideas and, naturally, make the most of other people's experiences, and work on future cooperation projects. It is never too late to be true to the urge hospital pharmacists typically feel to become involved in humanitarian aid and/or cooperation projects relative to one of the multiple disciplines covered by our specialty. The Spanish Cardiology Society recently set a wonderful example with their SECoopera project, aimed at promoting international cooperation in the field of cardiology10. We as hospital pharmacists could consider joining that initiative.
Thanks to the effort and engagement of the Sahrawi people and the assistance provided by MMMed, the Sahrawi refugee camp at Tindouf has a drug compounding laboratory. This is an important achievement for the RASD considering the duration of the project and the results obtained.
The laboratory makes available to the population a well-equipped facility, manned by well-trained staff, where low-complexity medications can be compounded. It also offers jobs that help further the professional and human development of the refugees living in the camps. The people that work at the laboratory have acquired the knowledge and skills needed to capably meet the challenges facing therm.
We hope the case presented in this article serves as inspiration for new projects aimed at supporting developing countries improve the healthcare available to their most disadvantaged population groups.
FundingWe would also like to acknowledge the contribution of the following institutions, organizations and entities:
Agencia Española de Cooperación Internacional, Numerosos Ayuntamientos de Cataluña, Ayuntamiento de Elche, Fundació Probitas, Asociación de trabajadores y Técnicos sin Fronteras (ATTsF), Rivas Sahel, Facultad de Farmacia de la Universidad de Barcelona, Farmacia Carreras de Barcelona, Hospital Clínic de Barcelona.
AcknowledgementsWe would like to acknowledge the help of the following people:
Hafed Didi, Jatri Said, Munaya Mohamed, Mohamed Ali, Mohamed Salem, Tikber Mohamed, Magali Mustafa, Magali Ahmed baba, Aziz Ali, Mohamed Salem, Chej Embarek, Ahmed Chej, Ehleila Babeh, Enguia Elbelal, Lamana Hamudi, Jueila Elmehdi, Brahim Ahmed, Salama Azman Ehmeida, Bachir Mohamed Salem, Bachir Saleh, Manna Busaula, Heya Elhusain, Majidi Bah, Mudi Mohamed, Sukaina Salma, Luarusi Dua, Rafael Hidalgo, Ángel Torres, Constança Alberti, Eulalia Pintó, Santi Grau, Luis Mendarte, Kiko Puigventós, Francesc Osorio, Carmen López, Laura Canadell, Patricia Domínguez, Ana Gómez, Alex Llovera, Jaime Serna, Iván Zainos, Mónica Vera, Ricardo Rodríguez, Viola Tomé, Consuelo Hernández, Ramón Ramón, José Antonio Ruiz, Victoria Luanco, Genis Castells, Montserrat Cofán, Núria Fernández, Pilar Iranzo, Sebastian Podlonik, Xavier Bosch, Toni Castells, María Teresa Alay, Carlos Codina, Elena del Cacho, Flaviano de Pablo, Marga García, Pau Mora, Vasco Coelho, José María Suñé, Miquel Carreras, M.a José Montejo, Isabel Mayoral, Pilar Pérez, David Gonzalo.
Conflict of interestNo conflict of interest.
Contribution to the scientific literature
This article describes the evolution of a pharmaceutical humanitarian assistance project based on building up and strengthening the healthcare infrastructure of a refugee camp. The goal is to report on an initiative aimed at providing such camps with the required infrastructure, equipment, and materials to compound medications with a view to improving accessibility of the population to the medications they need. The initiative also comprised the establishment of a training and occupational center managed by local staff.
Members of staff of the Embarek Fakal.la compounding laboratory.
Mohamed Lamin Abdi; Fatimetu Ahmed; Salama Azman; Mahfuda Mohamed; Brahim Ahmed; Mulay Masoud; Mohamed Salem; Jaditeyu Brahim; Tislem Sidali; Brahim Saleh; Hassena Salec; Hamdi Matala; Núria Fernández; Montserrat Cofan; Victoria Luanco; Genis Castells; Ma Elena del Cacho; Carlos Codina.
Early Access date (06/02/2021).