to develop the different factors involved in the physiopathology of trauma-induced coagulopathy, through a review of publications on the matter; as well as to assess the evidence available on the treatment of critical bleeding and the recommendations by clinical practice guidelines.
Methodsa search has been conducted on the bibliography published about the physiopathology and treatment of critical bleeding in the PUBMED, BestPractice, UpToDate databases and the Cochrane Plus Library. The main key words used for this search were “early trauma induced coagulopathy”, “mechanisms of early trauma-induced coagulopathy”, “blood transfusion guidelines”, “massive transfusion guidelines” and “fibrinogen replacement therapy”. The most clinically relevant articles were selected for this review.
Conclusionsthe physiopathology of the trauma-induced coagulopathy is a more complex matter and involves more factors than was initially assumed. The early treatment of the coa-gulopathy is critical for the initial management of the critical bleeding. However, the use of blood derivatives should be rational and based on homogeneous and high-quality scientific evidence.
The main cornerstones for the treatment of critical bleeding are: fluid therapy, fibrinogen concentrate, prothrombin complex concentrate, plasma, erythrocyte or platelet concentrates, tranexamic acid, and calcium. Their administration should be assessed depending on the clinical condition of each patient.
desarrollar los factores implicados en la fisiopatología de la coagulopatía asociada al traumatismo (CAT) mediante una revisión de la literatura publicada al respecto; ademas de revisar la evidencia disponible sobre el tratamiento de la hemorragia crítica y las recomendaciones de las guías de practica clínica.
Métodosse ha realizado una büsqueda de la bibliografía pu-blicada sobre la fisiopatología y tratamiento de la hemorragia crítica en las bases de datos PUBMED, BestPractice, UpToDate y la Biblioteca Cochrane Plus. Las principales palabras clave utiliza-das para la büsqueda han sido: “early trauma induced coagulopathy”, “mechanisms of early trauma-induced coagulopathy”, “blood transfusion guidelines”, “massive transfusion guidelines” y “fibrinogen replacement therapy”. Los artículos mas clínicamente relevantes han sido seleccionados para la revisión.
Conclusionesla fisiopatología de la coagulopatía asociada al traumatismo se trata de un cuadro mas complejo y multifac-torial de lo que inicialmente se había aceptado. El tratamiento precoz de la coagulopatía es imprescindible para el manejo ini-cial de la hemorragia crítica. No obstante, el uso de hemoderi-vados debería ser racional y basado en una evidencia científica homogénea y de alta calidad.
Los principales pilares del tratamiento de la hemorragia crítica son la fluidoterapia, el concentrado de fibrinógeno, el concen-trado de complejo protrombínico, el plasma, los concentrados de hematíes o de plaquetas, el acido tranexamico y el calcio. Su administración deberfa valorarse en función de las condiciones clfnicas de cada paciente.
A search was conducted on the bibliography published about the physiopathology and treatment of critical bleeding in the PUBMED, BestPractice database, UpToDate database, and the Cochrane Plus Library. The main key words used for this search were: “early trauma induced coagulopathy”, “mechanisms of early trauma-induced coagulopathy”, “blood transfusion guidelines”, “massive transfusion guidelines” and “fibrinogen replacement therapy”. The most clinically relevant articles have been selected for this review.
IntroductionCritical bleeding is the main cause of avoidable death after trauma. A fourth of all trauma patients will present a trauma-induced coagulopathy (TIC). Patients with TIC have a five times higher risk of death within the first 24 hours, higher transfusion requirements, a longer hospital stay, and are susceptible to presenting more com-plications1, Brohl2 and MacLoad3 had already stated in 2003 that trauma itself is the trigger for trauma-induced coagulopathy.
Within the setting of trauma patients, coagulopathies can be classified into two groups: TIC in the strict sense, or iatrogenic TIC. TIC is a pathologic response due to a deregulation of hemostasis, secondary to a trauma injury. Iatrogenic TIC, on the other hand, is caused by previous treatment with oral anticoagulants, or by he-modilution due to an abundant fluid therapy administered after the critical bleeding1.
Paradoxically, there are many similarities between disseminated intravascular coagulation with fibrinolytic phenotype (DIC) and TIC: low levels of fibrinogen (increased fibrinolysis and, therefore, increase in the fibri-nogen and fibrin degradation products), low platelet count, prolonged prothrombin time, and low levels of proteins controlling coagulation (e.g. antithrombin levels are lowered, and therefore, hypercoagulation will develop; or there is a reduction in the alpha2-plasmin inhibitor, leading to higher fibrinolysis)4.
Thus, the objectives of the present paper are to develop which mechanisms are involved in the physiopatho-logy of trauma-induced coagulopathy (TIC), through a review of literature published about this matter, as well as to review the evidence available on the treatment for critical bleeding and the recommendations by clinical practice guidelines.
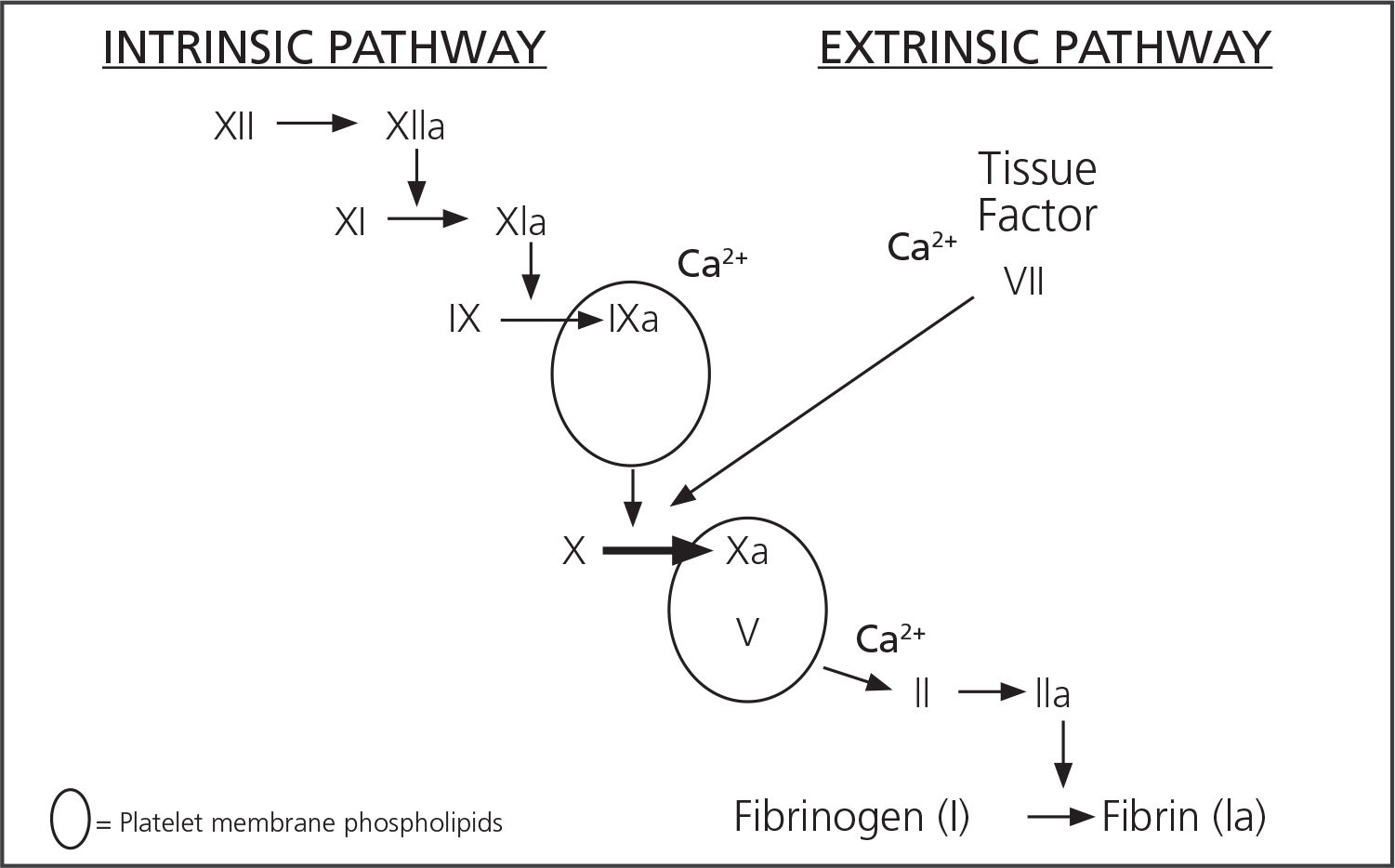
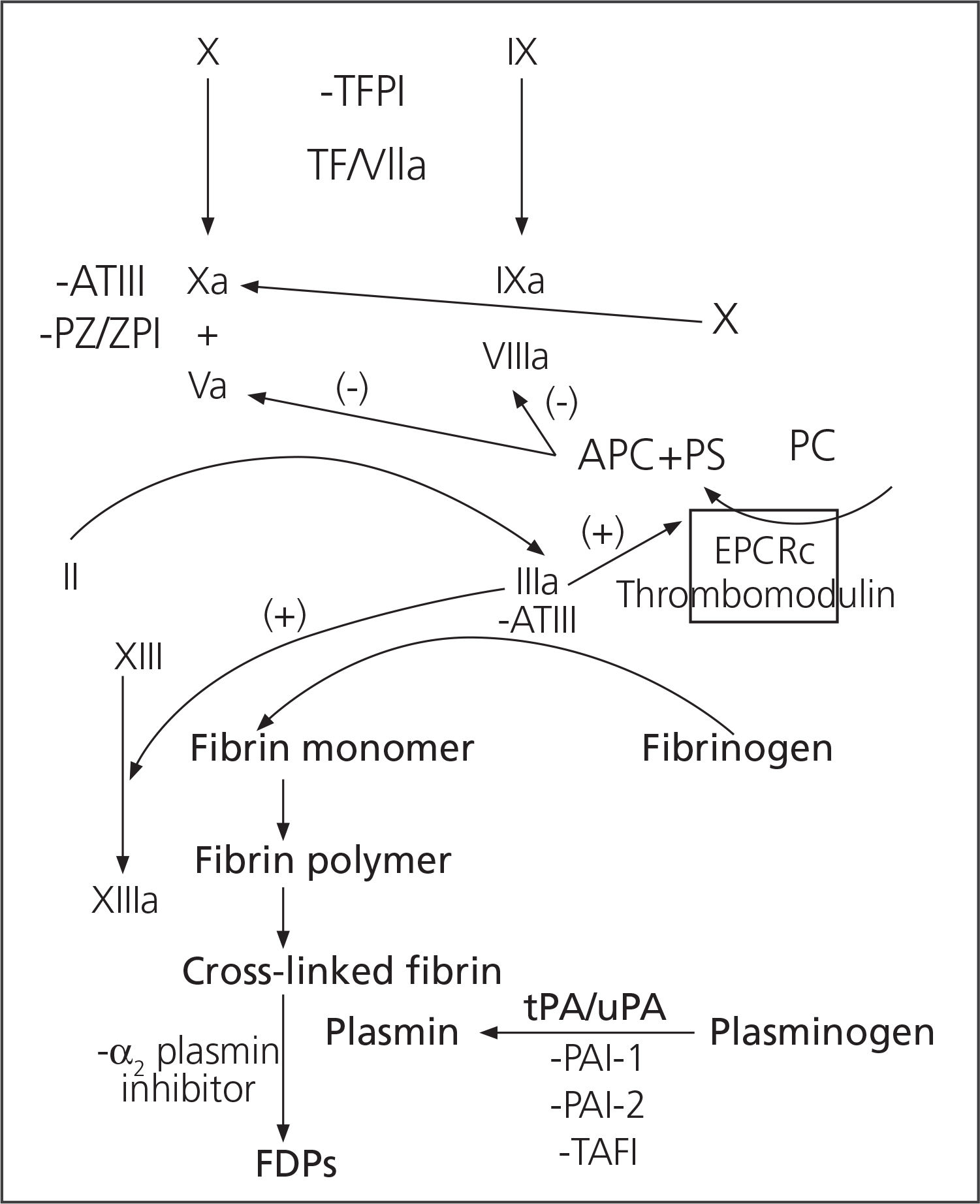
ResultsTic physiopathogenesisTraditionally, TIC mechanisms were focused on the he-modilution + hypothermia + acidosis triad. Though this triad is still valid, recent studies on the physiopathology of TIC, besides the Cell-based Model5,6, have demonstrated that this is a more complex matter which involves more factors than was initially assumed7 (Figure 1).
Other factors involved:
- -
Platelet dysfunction: The involvement of the platelet function was considered when sustained bleeding was observed in trauma patients with normal platelet counts. Multiple factors would be involved in platelet dysfunction: hypothermia (a consequence of bleeding and hemorrhagic shock), lesion severity, and the storage methods for platelet concentrates after donation by volunteers (media, temperatures, processing... ) It is believed that platelet dysfunction would also be affected as a response, for example, to ADP (adenosine dyphos-phate) arachidonic acid, collagen, and thrombin receptor activating peptide1, 8–10.
- -
Endothelial dysfunction: Some endothelial cells generate proteins, in the context of trauma, which will favour patient anticoagulation. These proteins inhibit thrombin formation through the production of thrombomodulin. With the activation of the protein C endothelial receptor, they will also produce chondroi-tin and heparan sulfate1, as well as a recently studied glycoprotein (sydecan-1)11. Chondroitin sulfate increases the efficiency of the thrombin inhibition conducted by the thrombomodulin; while heparin sulfate increases the efficiency of thrombin inhibition conducted by antithrombin III. Ostrowski et Johansson12 described an endogenous heparinization in 5% of those trauma patients studied, which corresponded to those patients with higher lesion severity, higher transfusion requirements, more prolonged prothrombin times, and higher evidence of endothelial damage.
- -
Protein C activation: Protein C plays a key role in TIC development. It presents a dual activity: cytoprotective and antiacogulant. On one hand, cytoprotective against cytotoxicity secondary to hypoperfusion after hemorrhagic shock, antiinflammatory and limiting endothelial permeability (higher permeability, higher inflammation and higher oedema); and on the other hand, anticoagulant inhibiting thrombin formation (inhibiting the FVa and FVIIIa factors) and promoting fibrinolysis (encouraging plasmin formation and inhibiting PAI-1, the physiological inhibitor of tPA and uPA, thus encouraging fibrin clot degradation and increasing the levels of fibri-nogen degradation products)1, 13,14.
- -
Oxidative modification of proteins involved in coagulation: It is believed that the oxidative modification of certain domains of proteins involved in coagulation, such as PAI-1, C protein, thrombomodulin or fibrin, would encourage TIC development15,16. Burney et al.17 have recently provided data about the oxidation of a methionine located in the alfa C-subdomain of fibrin. This results in the alteration of the fibrin lateral aggregation during polymerization, with the subsequent involvement of compact clot formation. This oxidative damage would appear in the setting of oxi-dative stress, such as hemorrhagic shock secondary to trauma. Reactive oxygen species are liberated by leukocytes, platelets and endothelial cells after the activation of inflammatory pathways, endothelial lesion and tissue hypoperfusion.
- -
Hyperfibrinolysis: The vast majority of multiple trauma patients will present a certain degree of fibrinolysis, and approximately 5% (according to Raza et al.18) would present severe hyperfibrinolysis. Fibrinogen degradation is controlled by plasmin, which is generated through plasminogen activation by tPA and uPA. Plasmin will degrade the cross-links between fibrin molecules, dissolving the fibrinogen clot. This fibrinogen degradation process is inhibited by PAI-1, which deactivates tPA and uPA. The relationship between activated protein C, which inhibits PAI-1, and hyperfibrinolysis, is reinforced. On the other hand, Lustenberger et al.19 have recently studied the TAFI (thrombin-activatable fibrinolysis inhibitor). This is a plasminogen activation inhibitor, and it has been observed to be reduced in patients with TIC. TAFI is activated by thrombin, and inhibits fibrinolysis by splitting a carboxy-terminal end of a lysine residue of the fibrin molecule to which plasminogen and tPA will bind (figure 2).
Early treatment for coagulopathy, together with fast diagnosis and the control of the source of bleeding, are three key points in the initial management of critical bleeding. The initial replacement strategy consists essentially in the administration of fibrinogen, prothrombin complex (PCC) and fresh frozen plasma (FFP)
FibrinogenThis is a glycoprotein synthesized in the liver, necessary both for platelet aggregation and fibrin formation. The conversion of fibrinogen to fibrin is catalyzed by thrombin, and fibrinogen levels will determine the quantity and complexity of the fibrin net formed during coagulation. If fibrinogen levels are reduced, the fibrin net will be more fragile and unstable, and will affect secondary hemostasis20–28.
On one hand, fibrinogen plays a key role in thrombus formation and stabilization; while it will also cause platelet activation and aggregation by binding with the GPIIb/IIIa platelet receptors. Low levels of fibrinogen have been associated with an increased risk of bleeding and a higher risk of mortality20–28.
Fibrinogen is the first plasmatic factor to become depleted in critical bleeding. There are three ways to supply fibrinogen: fresh frozen plasma, cryoprecipitate, and fibrinogen concentrate. The latter is the most frequently used, because it does not need refrigeration, does not require cross-tests, it does not cause hemodilution, and can be administered rapidly (up to 6g in under 3 minutes)26, 27.
Though clinical guidelines recommend fibrinogen administration in order to reduce bleeding and/or transfusion rate (level 1C)26,27,34, the indication according to label for fibrinogen concentrate administration is only for treatment of bleeding in patients with congenital hypo or afibrinogenemia with tendency to bleeding29.
There is no universal consensus regarding the critical levels of fibrinogen for trauma patients, though the key role of fibrinogen in critical bleeding control is widely accepted. Low fibrinogen levels are a negative prognostic factor, and therefore the early correction of levels has been associated with a higher survival. English30,31 and American32 guidelines recommend fibrinogen administration when levels are below 1g/L. On the other hand, the European Trauma Guidelines34, the European Society of Anaesthesiology35 and the Canadian National Advisory Committee on Blood and Blood Products33 recommend maintaining fibrinogen levels above 1.5-2g/L.
Prothrombin Complex Concentrate (PCC)Those PCCs marketed in Spain contain 4 coagulation factors (II, VII, IX and X). In order to minimize thrombogeni-city, they also contain protein C, protein S, antithrombin III and/or heparin. The different commercial brands are equally potent in terms of activity, but they have certain differences in terms of composition. Typically prescribed doses refer to factor IX, and are usually dosed at a mean 20UI/Kg (15-25UI/Kg)26,27,34,38.
Though the indications approved in the product speci-fications38 for PCCs are urgent reversal of anticoagulation by vitamin K antagonists (normalization of INR between 10 and 30 minutes after administration) and perioperative treatment and prevention of bleeding by congenital deficiency of any of the coagulation factors depending on vitamin K, its off-label use has become increasingly common in non-anticoagulated patients, trauma patients, or those who present uncontrolled bleeding during surgery36–37. In fact, clinical guidelines recommend its use in patients not treated with vitamin K antagonists (VKAs), with coagulo-pathy in the context of trauma, perisurgical bleeding, or acute liver impairment with a level 2C of evidence / recom-mendation26,27,34.
An increasing number of clinical guidelines are highlighting its numerous advantages vs. plasma. This is a concentrated product which does not worsen hemodilution or has any impact on the fluid balance of the patient. It can be stored at room temperature, and therefore does not require to be defrosted before administration. It can be administered regardless of blood type, with rapid administration and anticoagulation reversal. In intracranial hemorrhage, which is the most severe event associated with anticoagulation with vitamin K antagonists, studies have demonstrated a higher INR correction and bleeding control in patients treated with PCC vs. those treated with plasma39,40.
Regarding the reversal of new oral anticoagulants, neither clinical guidelines nor product specifications have formally recommended its use, because efficacy and safety data are still very limited. As there is some experimental evidence which seems to support the use of activated pro-thrombin complex concentrates (e.g. FEIBA®), recombinant Factor VIIa or PCCs, their use could be considered in cases where there is an urgent need to reverse anticoagulation, such as, for example, an emergency surgical procedure41–43.
PlasmaClinical guidelines recommend (level 1B) an initial dose of 10-15ml/Kg for initial management of critical bleeding26,27,34. Plasma contains both procoagulants (such as coagulation factors and fibrinogen) and coagulation cascade inhibitors; as well as other proteins such as albumin or immunoglobulins. But as has been previously mentioned, it presents certain disadvantages when compared with the fibrinogen concentrate or PCC separately. There are no special storage conditions for any of both concentrates, there is no need to defrost, no compatibility tests are required, they can be administered rapidly with the subsequent rapid effect, and they do not cause the hemodilution generated by plasma. Reviews on the clinical utility of plasma, and studies comparing its use vs. fibrinogen concentrate or PCCs, have questioned the use of plasma for the management of critical bleeding, due to the advantages presented by the latter products44–46.
Erythrocyte and Platelet ConcentratesThe administration of Erythrocyte Concentrates (ECs) and Platelet Concentrates will be mostly conducted based on test results. It is not recommended to use hematocrit as an isolated marker for critical bleeding (level 1B). However, the administration of erythrocyte concentrates is recommended in order to maintain hemoglobin levels between 70 and 90mg/L (level 1C)26,27–34.
Primary hemostasis is well preserved with platelet levels of 100x109 platelets/L, as long as platelet function is adequate. It is recommended to administer platelet concentrates in order to maintain levels above 50 x109 platelets/L (level 1C) to prevent thrombocytopenia from contributing to the bleeding26,27–34. Thrombocytopenia will typically develop after factor deficiency (fibrinogen, prothrombin complex...) and after the clinical development of microvascular hemorrhage.
It is known that erythrocytes are involved in hemostasis by stimulating platelet activation and thrombin genera-tion47, and that hematocrit levels below 30% (Hb 9g/dl) will reduce the hemostatic efficacy of platelets. However, more studies are necessary in order to determine the he-matocrit and haemoglobin levels required for controlling a massive bleeding.
Tranexamic Acid (TXA)European clinical guidelines for management of critical bleeding in trauma patients recommend the use of antifi-brinolytics (level 1B) in patients with hyperfibrinolysis26,27,34. In the Update of the Seville Document, treatment with tra-nexamic acid (TXA) is recommended in order to reduce bleeding and/or transfusion rate in multiple trauma patients with significant bleeding (level 1B)26,27.
Antifibrinolytics include TXA and Epsilon-aminocaproic acid: both are synthetic lysine analogues that competitively inhibit plasminogen binding to lysine residues on the fibrin surface, thus preventing the conversion of plasmino-gen into plasmin. TXA is 10 times more potent than Epsi-lon-aminocaproic acid. The recommended doses for TXA are 10-15mg/Kg followed by a 1-5mg/kg/h infusion; the Epsilon-aminocaproic acid doses are 100-150mg/Kg followed by a 15mg/Kg/h infusion26,27.
Specifically, TXA is a very cost-effective drug, which has demonstrated in recent studies a reduction in the incidence of coagulopathy and in mortality48,49 (Table 1).
Some clinically relevant publications about the use of hemoderivatives in critical bleeding
| Hemoderivative assessed | Article | Contents |
|---|---|---|
| Fibrinogen | Farriols et al.56 | 69 patients were included in this observational and retrospective study; 62% of them presented hypofibrinogenemia due to consumptive coagulopathy. After a mean dose of 4g, a mean increase of 1.09g/L was observed in plasma fibrinogen. Coagulation parameters increased significantly (P < 0.001). Mortality rates were 32.3% (after 24 hours), and 44.2% (after 72h). Through logistic regression, a relationship was established among patients with an acute fibrinogen deficiency between higher levels of plasma fibrinogen and a higher survival (P = 0.014). In the rest of more chronic hypofibrinogenemias, only a trend was observed, without being significant. |
| Fibrinogen | The Cochrane Collaboration28 | A systematic search was conducted in the following databases: the Cochrane Central Register of Controlled Trials; MEDLINE (from 1950 to August, 9th, 2013); EMBASE (from 1980 to August, 9th, 2013); International Web of Science (from 1964 to August, 9th, 2013); CINAHL (from 1980 to August, 9th, 2013); LILACS (from 1982 to August, 9th, 2013); and in the Chinese Biomedical Literature Database (until November, 10th, 2011); together with the databases for on-going clinical trials. All randomized clinical trials were included, which compared fibrinogen concentrate with placebo or another treatment in bleeding patients, excluding newborns and hereditary disorders. It was observed that the fibrinogen concentrate seems to reduce transfusion requirements, but those clinical trials included lacked the potency to detect differences regarding mortality or clinical benefit. Overall, the conclusion is that the evidence supporting the use of fibrinogen in critical bleeding is weak and heterogeneous, and that further research about it is required. |
| Fibrinogen vs. plasma | Kozek-Langenecker et al.45 | Those studies that assessed blood loss, transfusion requirements, duration of hospital stay, survival, and plasma levels of fibrinogen when the fibrinogen concentrate or fresh plasma were used, were identified from databases (from 1995 to 2010). Scientific evidence does not seem to favour the use of plasma in the surgical setting, or for trauma patients with massive transfusion. The administration of the fibrinogen concentrate was generally associated with better outcomes. |
| PCC | Leal-Noval et al.36 | 142 patients treated with PCC were included in this observational and retrospective study. Patients were classified into three groups: those anticoagulated with VKA (receiving surgery or at risk of critical bleeding), anticoagulated with VKA and with intracranial bleeding, and non-anticoagulated patients at risk of critical bleeding. All patients received a mean dose of 1200UI (15UI/Kg) of PCC, and their INR was reduced from 4 ± 3 to 1.7 ± 1.2 (P < 0.01). INR normalization was significant in the three arms, but particularly in those with INR > 4. Blood loss and transfusion requirements were also significantly reduced in the three arms. |
| TXA | CRASH-2 trial collaborators48 | Blind and randomized clinical trial conducted in 274 hospitals from 40 countries. 20211 adult trauma patients at risk or with critical bleeding were included. These were randomized within the 8 hours after admission to TXA (loading dose of 1g in 10min, followed by 1g every 8 hours), or to placebo. TXA reduced mortality (for any cause) safely and significantly, as well as the risk of bleeding. Mortality in the TXA group: 1463 [14-5%] vs placebo: 1613 [16-0%]; relative risk 0-91, 95% CI 0-85-0-97; p = 0-0035. The risk of death due to critical bleeding was reduced from the TXA arm [4-9%] vs. placebo 574 [5-7%]; relative risk 0-85, 95% CI 0-76-0-96; p = 0-0077). |
PCC (Prothrombin Complex Concentrate); VKA (Vitamin K antagonists); TXA (Tranexamic Acid).
Calcium is essential for the activation of coagulation factors in different stages, therefore it is necessary to maintain adequate levels of this cation. Guidelines recommend with an evidence / recommendation level 1C to monitor calcium levels in plasma in order to maintain them >0,9 mmol/l (therapeutic range 1.1-1.3 mmol/l) during massive transfusion. Low levels of plasma calcium at admission have been associated with a higher transfusion requirement and higher mortality. However, there are no data demonstrating that preventing hypocalcemia can reduce mortality among patients at risk of critical bleeding34.
Activated Factor VII (rFVII)Its authorization in Europe extends to patients with selective factor VII deficiency and Glanzmann thrombas-thenia. Its off-label use has been relegated, in patients with bleeding refractory to surgical hemostasis and usual hemotherapy support, as previously mentioned (level 2C)26,27,34. It will only be effective if the sources of active bleeding are controlled, and if the levels of fibrinogen, platelets, hematocrit, plasma calcium, plasma pH, etc., have been minimally normalized34.
A systematic review50 where rFVII was assessed in 5 indications (intracranial hemorrhage, cardiac surgery, trauma, liver transplant, and prostatectomy) concluded that there is no evidence for reduction in mortality with rFVIIa, and that in some of the indications, such as intracranial hemorrhage after traumatic brain injury, it increased the risk of thromboembolism. That it why there is a recommendation against its use in this indication, with a level 2C26,27,34.
Fluid TherapyClinical guidelines recommend an early initiation of fluid therapy in those patients with active bleeding and hypotension (level 1A). Hypovolaemia correction through fluid administration is the first measure to take for any type of severe bleeding, because the body has a much lower tolerability to hypovolaemia than to anae-mia26,27.
It is recommended that crystalloids should represent the initial option for volemia restoration (level 1B). The most widely used crystalloids are: isotonic saline solution at 0.9%, Ringer’s solution, and other “balanced” solutions (Plasmalyte®, containing acetate; Ringer’s lactate®, containing lactate). The lactate in the latter can be metabolized into bicarbonate, and in theory could be useful to treat the metabolic acidosis of the lethal triad in these patients; but lactate metabolism is inhibited during shock. On the other hand, Ringer’s lactate has been considered a more physiological source of chloride (109mmol/L) than isotonic saline solution at 0.9% (150mmol/L). Some studies51 have even suggested the association between the use of fluid therapy regimens restricted in chloride and a lower incidence of renal impairment and requirement for renal replacement, but results have not been conclusive. That is why, in order to reduce the risk of metabolic disorders during resuscitation or volemic reposition, it is recommended that balanced saline solutions (Ringer’s lactate® or acetate) should replace normal saline at 0.9% (level 1B); as long as there is no traumatic brain injury26,27.
The main adverse effect of resuscitation with crystalloids is dilutional coagulopathy. For this reason, when crystalloids are not enough, the use of colloids should be assessed. Those colloids available are: hydroxyethyl starches, gelatins, and human albumin. The disadvantages of gelatins are their low molecular weight, their limited ability to expand (70-80%), and their short half-life (2-3 hours). Albumin has a major volume expanding effect (albumin 5%: 100%; albumin 20%: 200-400%) with fast onset of action and sustained action, but its use is not recommended in the context of the bleeding patient. The problem it presents is that it is not contained exclusively in the intravascular space, and it could worsen the interstitial and pulmonary oedema; at the same time, it could cause coagulation disorders and hemostasis by inhibiting platelet function and increasing the effect of antithrombin III, leading to a state of hypocoagulability. Besides, this is a hemoderivative, with their associated problems (high price, limited source of supply, etc.) Therefore, the solutions that contain hydroxyethyl starches are the most widely used for volume expansion when the single infusion of crystalloids is not considered enough. These must be used at the minimal effective doses and during the shortest period of time possible.
After the safety warnings published in 2013, the European Pharmacovigilance Risk Assessment Committee (PRAC) confirmed that those solutions for intravenous perfusion that contain hydroxyethyl starch must not be used in patients with sepsis, patients in critical condition, or burnt patients, due to a higher risk of severe renal failure and higher mortality. These solutions will only be indicated in case of hypovolemia due to acute bleeding, for a maximum of 24 hours, and watching renal function during at least 90 days, as long as treatment with crystalloid solutions is not considered enough, and taking into account all contraindications and precautions for use52–55.
Thromboelastography (TEG): Bedside management of coagulationTEG assesses the viscoelastic properties of coagulation during clot formation and lysis. This technique represents an advance in coagulopathy diagnosis, because it allows to make early bedside clinical decisions. In less than 10 minutes, results are obtained graphically, allowing a fast assessment of overall coagulation, and to replace those coagulation factors that the patient is really lacking26,27.
DiscussionIt is worthwhile knowing the physiopathology of trauma-induced coagulopathy, because critical bleeding is the main cause of avoidable death after trauma. Besides, in the majority of cases the patient is a healthy individual who could return to their basal situation once they have overcome the coagulopathy, and the trauma if possible. Therefore, it is important also to know in which conditions the use of hemoderivatives will be optimal for critical bleeding management.
Both the hemodilution, hypothermia and acidosis triad used to explain TIC, as the traditional coagulation cascade clearly differentiated between the intrinsic and extrinsic pathways, are very useful from an educational point of view in order to understand the physiopathology of critical bleeding, but they have been gradually relegated. In the new theories, TIC physiopathology would be much more complex, with many more interrelated factors involved.
Undoubtedly, it is also necessary to continue studying which conditions of use are optimal for each hemoderivative, in order not to use them indiscriminately. On one hand, current evidence supporting the use of said hemoderivatives is weak and of low quality. On the other hand, there is a certain wrong perception of the innocuousness of hemoderivatives, regardless of their associated problems. These are not free from adverse reactions (anaphylactic reactions, increased risk of thromboembolism, dilutional coagulopathy, etc.), from a minimal risk of infectious disease transmission, limited sources of supply, and a high economic cost upon hospital budgets.
ConclusionsTrauma-induced coagulopathy is a multifactorial condition, and the components of the coagulation cascade are much more interrelated than was traditionally believed.
Early treatment of coagulopathy is essential for the initial treatment of critical bleeding. However, there should be a rational use of hemoderivatives. The benefit-risk balance should be individually assessed and justified by biochemical tests, blood count test, or throm-boelastography.
More studies are required in order to homogenize the recommendations by clinical guidelines, and determine when it will be optimal to use each hemoderivative in the context of trauma-induced critical bleeding.