The aim of this study was to perform an adjusted indirect treatment comparison, according to the cytogenetic profile, in terms of efficacy between different Bruton tyrosine kinase inhibitors used as first-line monotherapy for chronic lymphocytic leukemia. Safety outcomes considered of interest were also evaluated to establish whether these options can be considered equivalent therapeutic alternatives.
MethodA literature search was conducted in Pubmed and Embase on November 10, 2022 for phase III clinical trials studying Bruton tyrosine kinase inhibitors in monotherapy in the first-line setting for chronic lymphocytic leukemia. Results were filtered according to whether the combination of bendamustine and rituximab was used as comparator and whether they had similar populations and follow-up times. Subgroup results were meta-analyzed according to mutational characteristics by classifying patients into high and low cytogenetic risk. An adjusted indirect comparison was developed using Bucher's method. Possible therapeutic equivalence was determined by applying the guide to equivalent therapeutic alternatives.
ResultOf the 39 studies obtained in the review, 2 clinical trials were selected: 1 for zanubrutinib and 1 for ibrutinib. The remaining studies were not included because they did not meet the inclusion criteria. The results obtained in the adjusted indirect treatment comparison for both cytogenetic risk subgroups showed no statistically significant differences. The most relevant safety differences were atrial fibrillation, hypertension, and cardiovascular events in patients treated with ibrutinib and higher incidence of secondary cancers in patients treated with zanubrutinib. Applying the equivalent therapeutic alternatives guideline criteria, both treatments cannot be considered equivalent therapeutic alternatives.
ConclusionsAssuming the uncertainty associated with the adjusted indirect comparison, zanubrutinib could be considered equivalent in efficacy to ibrutinib, however, the presence of differentiating safety features precludes assigning the 2 alternatives as equivalent therapeutic alternatives.
El objetivo del presente trabajo fue realizar una comparación indirecta ajustada, según el perfil citogenético, en términos de eficacia, entre los distintos inhibidores de la tirosin quinasa de bruton empleados como monoterapia en primera línea para la leucemia linfocítica crónica. Asimismo, se evaluaron los resultados de seguridad considerados de interés para establecer si dichas opciones pueden ser consideras alternativas terapéuticas equivalentes.
MétodoCon fecha 10 de noviembre del 2022, se llevó a cabo una búsqueda bibliográfica en las bases de datos de Pubmed y Embase de ensayos clínicos fase III que estudiaran los inhibidores de la tirosin quinasa de Bruton en monoterapia en contexto de primera línea para la leucemia linfocítica crónica. Se incluyeron ensayos en los que se empleara la combinación de bendamustina y rituximab como comparador y que presentaran poblaciones y tiempos de seguimiento semejantes. Se combinaron mediante metanálisis los resultados de los subgrupos según las características mutacionales clasificando a los pacientes en alto y bajo riesgo citogenético. Se desarrolló una comparación indirecta ajustada utilizando el método de Bucher. Se determinó la posible equivalencia terapéutica aplicando para ello la guía de alternativas terapéuticas equivalentes.
ResultadoDe los 39 estudios obtenidos en la revisión, se seleccionaron 2 ensayos clínicos: uno para zanubrutinib y otro para ibrutinib. El resto de estudios no se incluyeron por incumplimiento de los criterios de inclusión. Los resultados obtenidos en la comparación indirecta ajustada para ambos subgrupos de riesgo citogenético no mostraron diferencias estadísticamente significativas. En cuanto a la seguridad, las diferencias más relevantes se encontraron en la incidencia de fibrilación auricular, hipertensión arterial y eventos cardiovasculares en los pacientes tratados con ibrutinib, y mayor incidencia de cánceres secundarios en los pacientes tratados con zanubrutinib. Aplicando los criterios de la guía ATE, ambos tratamientos no podrían ser considerados alternativas terapéuticas equivalentes.
DiscusiónAsumiendo la incertidumbre asociada a la comparación indirecta ajustada, zanubrutinib podría ser considerado de similar beneficio clínico en eficacia a ibrutinib, sin embargo, la presencia de características diferenciadoras en el perfil de seguridad impide asignar a ambas alternativas como alternativas terapéuticas equivalentes a todos los efectos.
Chronic lymphocytic leukemia (CLL) is a hematological disease characterized by the proliferation and accumulation of malfunctioning mature B-cells in blood and tissues, including the bone marrow and lymphoid tissues. CLL cause the so-called B-symptoms, along with adenopathies, anemic syndromes, and infections.1 CLL mainly affects elderly individuals, most frequently male, at a ratio of 2:1.2 This disease is the most frequent form of leukemia in Western countries, accounting for 30% of cases.3
The American Cancer Society estimated that 20 .160 new patients would be diagnosed of CLL in 2022.4
The selection of the therapeutic armamentarium will depend on a range of cytogenetic factors evaluated at diagnosis, including 17p deletion (del[17p]), TP53 gene mutation, 11q deletion (del[11q]), and the mutational status of immunoglobulin heavy chains (IgHV), added to patient's age and functional status. There is a variety of chemotherapy and immunochemotherapy schemes available as first-line treatment, added to Bruton's tyrosine kinase inhibitors (iBTK) and BCL-2 inhibitors.5,6
Ibrutinib was the first iBTK approved by the European Medicine Agency (EMA) as a result of the Rosanate-2 study, which compared the effectiveness of iBTK vs chlorambucil. This 8th-year follow-up study revealed a benefit of iBTK in progression-free-survival (PFS), as compared to chlorambucil in monotherapy, with a hazard ratio (HR) of 0.154 (95% CI 0.108 to 0.220).7 In 2020, the ELEVATE-TN trial demonstrated the efficacy and safety of acalabrutinib, the first next-generation iBTK administered in combination or not with obinutuzumab, vs chlorambucil–obinutuzumab, as first-line treatment of CLL. Acalabrutinib, used either in combination with obinutuzumab or alone, was superior to the comparator in improving PFS. However, no benefit was observed in terms of overall survival (OS).8
The phase III SEQUOIA trial demonstrated a benefit of zanubrutinib in PFS, but not in OS, as compared to bendamustin–rituximab (BR).9
The emergence of this new iBTK makes it necessary to assess whether there are relevant differences in efficacy among the different iBTK currently available that prove one to be superior to the others as first-line treatment. Matching-adjusted indirect comparisons (MAICs) based on the use of a common comparator show the relative efficacy of 2 or more drugs that have not been directly compared in any randomized clinical trial. The clinical relevance of the differences observed in a MAIC analysis is assessed based on a delta (Δ) value (maximum difference considered clinically relevant). This method helps determine whether the drugs analyzed are therapeutically equivalent drugs (TED),10 i.e. whether they are therapeutic options with similar clinical benefits.
A MAIC analysis based on the cytogenetic profile of patients was performed to compare the efficacy of the different iBTK used as first-line monotherapy for CLL, using BR as the common comparator. The purpose was to determine whether the different iBTK available can be considered TED.
MethodsLiterature search and inclusion criteriaA literature search was carried out on Pubmed and Embase of phase III clinical trials assessing the use of iBTK alone as first-line treatment of CLL. The filters used included clinical queries (clinical queries), narrow, Clinical Trial Phase IIIon Pubmed; and Publication types—article, Clinical Trial Phase IIIon Embase. Keywords included: (ibrutinib OR acalabrutinib OR zanubrutinib) AND chronic lymphocytic leukemia AND (untreated OR treatment-naive OR naive OR first-line OR first-line treatment). An analysis of references (citation tracking) and a non-systematic search of websites were also performed. The studies included used BR as a common comparator and involved matched populations and similar follow-up periods. The primary endpoint was PFS, defined as the period from randomization to disease progression or death (whatever occurred first). Studies not providing survival data were excluded.
Data analysisA subgroup metaanalysis was carried out to categorize patients by their cytogenetic profiles as high risk (patients with del17p, TP53 mutation, del11q, and/or no IgHV mutations) and low risk (no del17p, TP53 mutation, del11q, and/or IgHV mutations), using the calculator developed by Joaquín Primo's.11 Heterogeneity and consistency were assessed using the Q-test.
A MAIC analysis of the different iBTK available was performed using Bucher's method and the calculator developed by the Canada's Drug and Health Technology Agency. Assessment of iBTK12,13 as drugs with similar clinical benefit was performed in accordance with TED guidelines.10 This method establishes criteria to assess whether 2 or more drugs can be considered therapeutically equivalent drugs in terms of efficacy. According to these guidelines, for 2 or more drugs to be considered TED, it is necessary to use robust evidence (including high quality studies only); use the most clinically relevant outcome and consider whether it entails severe or irreversible damage to the patient; and establish a margin of clinical relevance (Δ value). The Δ value is defined as the maximum difference considered clinically irrelevant across the different therapeutic options explored. In our study, we used the Δ value established by panels of experts, estimated on the European Medical Oncology Society's Magnitude of Clinical Benefit Scale (ESMO-MCBS).14 The ESMO-MCBS is a standardized application for comparing the clinical benefit of different cancer treatment options based on data from distinct randomized clinical trials. This tool is based on a form that assesses the curative intent of each treatment, the primary endpoint used, the type of clinical trial, and outcomes assessed in terms of quality of life, toxicity, and improved survival, as compared to a common comparator.15
Outcomes were represented graphically to determine whether the HR and the 95% confidence interval obtained in MAIC analysis fell within the Δ margin. The calculator developed by Shakespeare et al. was used to determine the probability that the outcome exceeded the margin of equivalence.16
In accordance with TED guidelines, for 2 or more agents to be considered TED, toxicity must also have been assessed in their respective clinical trials. The purpose is to detect any substantial difference that prevents the drugs studied from being considered TED. The frequency of grade 3–4 adverse drug reactions (ADRs) was compared to determine whether iBTK were also TED in terms of safety.
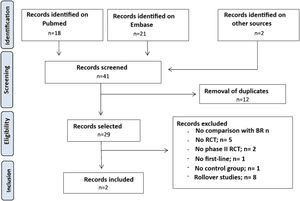
ResultsLiterature searchA literature search was conducted on Mediline (Pubmed) and Embase for studies published until November 10, 2022. Details are shown in Fig. 1. A total of 39 studies were retrieved. Abstracts were screened, and the 2 studies found to be potentially eligible underwent full-text reading: a study on ibrutinib and another on zanubrutinib.
An analysis of bibliographic references and a complementary search on the use of iBTK in naive patients with CLL were conducted on November 11, 2022. Although 2 new randomized clinical trials (RCT) were detected, they were finally excluded, as they did not meet eligibility criteria.
Finally, the RCTs A041202 (ibrutinib) and SEQUOIA (zanubrutinib) studies were included for MAIC analysis. Acalabrutinib was excluded due to the lack of studies that used BR as a comparator. The 2 RCTs included patients older than 65 years. Of note, the SEQUOIA study also included patients >18 years with comorbidities, who were not candidates to the fludarabine, cyclophosphamide, and rituximab scheme because they had a score >6 on the cumulative illness rating scale and a creatinine clearance <70 ml/min.
The A041202 (N = 547) study was a double-blind phase III RCT study comparing the use of ibrutinib alone and ibrutinib in combination with rituximab vs BR (to a 1:1:1 ratio) in naive patients with CLL. The median age was 71 years, with 97% of patients having an ECOG 0–1. The frequency of del17p, TP53 mutation, del11q, and no IgHV mutations was 9%, 19%, 63%, and 54%, respectively, in the arm of ibrutinib in monotherapy. The SEQUOIA study (N = 590) was a double-blind, phase III RCT comparing zanubrutinib against BR, to a 1:1 ratio, in patients with CLL without del17p. The study included a third arm of patients with del17p who received zanubrutinib, which was not compared with the control group. The median age was 70 years, with 94% of patients having an ECOG 0–1. The frequency of del17p, TP53 mutation, del11q, and no IgHV mutation was 6%, 18%, 53%, and 29%, respectively in the arm of zanubrutinib in monotherapy.
Data analysisBoth, ibrutinib (HR 0.39 [95% CI 0.26 to 0.58]) and zanubrutinib (HR 0.42 [95% CI 0.28 to 0.63]) demonstrated to be statistically superior to their BR comparator in terms of PFS. With respect to OS, no statistically significant differences were observed between the 2 iBTK. Table 1 shows the PFS reported in the 2 RCTs for ibrutinib and zanubrutinib, expressed as HR and its 95% CI, in subgroups of patients categorized by their cytogenetic characteristics.
Efficacy demonstrated in each treatment arm according to their cytogenetic characteristics, and final metaanalysis.
| Low cytogenetic risk patients | High cytogenetic risk patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meta-analysisHR (95% CI) | Mutated IgHVHR (95% CI) | Absence of TP53 mutationHR (95% CI) | Absent del11qHR (95% CI) | Absence of del17pHR (95% CI) | RCT | Presence of del17pHR (95% CI) | Presence of del11qHR (95% CI) | Presence of TP53 mutationHR (95% CI) | Non-mutated IgHVHR (95% CI) | Meta-analysis:HR (95% CI) |
| 0.44 (0.27 to 0.72) | – | – | 0.44 (0.27 to 0.72) | A041202 (Ibrutinib) | 0.26 (0.12 to 0.56) | – | – | 0.26 (0.12 to 0.56) | ||
| 0.47 (0.36 to 0.63) I2 = 13% | 0.67 (0.36 to 1.22) | 0.38 (0.25 to 0.59) | 0.50 (0.32 to 0.80) | – | SEQUOIA(zanubrutinib) | – | 0.21 (0.09 to 0.50) | 1.19 (0.28 to 4.99) | 0.24 (0.13 to 0.43) | 0.27 (0.17 to 0.43) I2 56% |
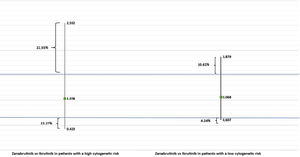
Clinical relevance was assessed using the HR 0.65 (and its inverse 1.54) as the Δ value. Fig. 2 contains a graphical representation of the results of our MAIC analysis. No statistically significant differences were observed between the 2 iBTK in the 2 subgroups of patients. In addition, the HR value did not exceed the Δ value established; therefore, it was concluded that the 2 agents were probably equivalent in terms of efficacy, in accordance with TED guidelines. Based on Shakespeare et al. calculator, the 95% CI of the MAIC analysis between zanubrutinib and ibrutinib in patients with high cytogenetic risk was 22.55% greater and 13.27% lower than the margin of equivalence, respectively. In contrast, in patients with a low cytogenetic risk, the 95% CI was 10.26% greater and 4.24% lower than the margin of equivalence, respectively.
With respect to safety, a MAIC analysis could not be performed due to the lack of agreement on the definition of ADR. Table 2 contains the frequency of grade 3–5 adverse drug reactions reported in the RCTs included. The A041202 study revealed statistically significant differences between ibrutinib and the BR control group in the percentage of patients who experienced atrial fibrillation, arterial hypertension, and cardiovascular events. In the SEQUOIA study, the frequency of secondary tumors was significantly higher in the control group, as compared to the zanubrutinib group. Likewise, neutropenia and thrombocytopenia were common ADRs, with significant differences between the 2 iBTK and the comparator BR.
Demonstrated grade 3–5 ADRs per treatment arm in the randomized clinical trials selected.
| Grade 3 to 5 ADR | Clinical trial | Treatment | No. of events (%) | ARR (95% CI) | P |
|---|---|---|---|---|---|
| Bleeding | A041202 | Ibrutinib | 3 (2) | −2.00 (−4.05 to 0.05) | >0.05 |
| BR | 0 (0) | ||||
| SEQUOIA | Zanubrutinib | 9 (3.8) | −2.00 (−4.97 to 0.97) | >0.05 | |
| BR | 4 (1.8) | ||||
| Neutropenia | A041202 | Ibrutinib | 27 (15) | 25.00 (16.08 to 33.92) | <0.05 |
| BR | 71 (40) | ||||
| SEQUOIA | Zanubrutinib | 27 (12) | 39.00 (31.31 to 46.69) | <0.05 | |
| BR | 110 (51) | ||||
| Anemia | A041202 | Ibrutinib | 21 (12) | 0.00 (−6.75 to 6.75) | >0.05 |
| BR | 22 (12) | ||||
| SEQUOIA | Zanubrutinib | 1 (0.4) | 1.40 (−0.51 to 3.31) | >0.05 | |
| BR | 4 (1.8) | ||||
| Thrombocytopenia | A041202 | Ibrutinib | 12 (7) | 8.00 (1.54 to 14.46) | <0.05 |
| BR | 26 (15) | ||||
| SEQUOIA | Zanubrutinib | 4 (1.7) | 5.30 (1.60 to 9.00) | <0.05 | |
| BR | 16 (7) | ||||
| Secondary tumors | A041202 | Ibrutinib | 10 (6) | −2.00 (−6.52 to 2.52) | >0.05 |
| BR | 7 (4) | ||||
| SEQUOIA | Zanubrutinib | 17 (7.1) | −4.00 (−7.95 to −0.05) | <0.05 | |
| BR | 7 (3.1) | ||||
| Infections | A041202 | Ibrutinib | 37 (20) | −5.00 (−12.87 to 2.87) | >0.05 |
| BR | 26 (15) | ||||
| SEQUOIA | Zanubrutinib | 39 (16.3) | 2.60 (−4.31 to 9.51) | >0.05 | |
| BR | 43 (18.9) | ||||
| Cardiovascular | A041202 | Ibrutinib | 29 (25) | −21.00 (−27.96 to −14.04) | <0.05 |
| BR | 7 (4) | ||||
| SEQUOIA | Zanubrutinib | 26 (10.7) | −2.10 (−7.45 to 3.25) | >0.05 | |
| BR | 23 (8.6) | ||||
| Arterial hypertension | A041202 | Ibrutinib | 53 (29) | −14.00 (−22.47 to −5.53) | <0.05 |
| BR | 25 (15) | ||||
| SEQUOIA | Zanubrutinib | 15 (6) | −1.00 (−5.13 to 3.13) | >0.05 | |
| BR | 11 (5) | ||||
| Atrial fibrillation | A041202 | Ibrutinib | 17 (9) | −6.00 (−10.88 to −1.12) | <0.05 |
| BR | 5 (3) | ||||
| SEQUOIA | Zanubrutinib | 1 (0.4) | 0.90 (−0.78 to 2.58) | >0.05 | |
| BR | 3 (1,3) |
According to TED guidelines, and based on the results of our MAIC analysis regarding the efficacy of ibrutinib vs zanubrutinib, along with the safety results of the RCTs, the 2 iBTK cannot be considered therapeutically equivalent options for first-line treatment in patients >65 years with CLL.
DiscussionThe emergence of novel iBTK for naive patients with LCC represents a change of paradigm in therapeutic decision-making. In the absence of direct comparative studies, MAIC analyses, along with network meta-analyses, emerge as an optimal tool for determining the most effective and safe therapeutic option among the treatments available.
Therapeutic decision-making is expected to be performed in the future as a function of the cytogenetic characteristics of patients. The prognostic model that categorizes patients by their cytogenetic risk is a pragmatic approach widely used in clinical practice in the selection of the most appropriate treatment.17 In February 2022, the Spanish Agency for Medicines and Medical Devices (AEMPS) published a positioning statement in relation to the combination therapy of venetoclax plus obinutuzumab in cancer patients. The different therapeutic options are described according to the presence or absence of cytogenetic risk.6 It is necessary to assess potential differences in the efficacy variables evaluated in RCTs to determine whether a therapeutic option is superior to others in these subgroups of patients.
The TED guidelines help evaluate, interpret, and determine the place in therapeutics of 2 or more therapeutic options available for the same indication. This way, researchers can assess clinically relevant differences between the range of therapeutic options available and categorize them as equivalent or not. The identification of therapeutically equivalent drugs facilitates price competition in procurement processes, thereby ensuring the sustainability of health systems.10 For these guidelines to be applied, it is necessary to establish an appropriate Δ value, as the validity of the study will depend on said value. Based on this premise, the Δ value was established in our study taking ESMO-MCBS scoring as a reference. ESMO-MCBS is a validated, widely accepted scoring system that is currently used to facilitate decision-making for the prioritization of therapies in cases of solid tumors.14
According to the results obtained, there are no objective criteria that prove the superiority of a drug over the other in terms of efficacy in different subgroups of cytogenetic risk. This conclusion is based on 2 premises: (i) it was assumed that treatment failure would involve disease progression. However, the results obtained in different clinical trials reveal that there is not a direct relationship between disease progression and a loss of overall survival in the long-term. (ii) The results of the calculator developed by Shakespeare et al. show that the highest proportion of the 95% CI did not exceed the margin of equivalence established, especially in the subgroup of patients with a low cytogenetic risk. Notably, the proportion exceeding the Δ value was higher among patients with a high cytogenetic risk, partially due to the high level of uncertainty of indirect comparison.
However, the differences observed regarding cardiovascular safety should be considered when evaluating the 2 treatments using TED guidelines. These results are consistent with the new safety notice published by the Spanish Ministry of Health on the higher incidence of cardiovascular events associated with ibrutinib in patients older than 65 years with previous cardiac diseases.18
In December 2022, Brown et al. published a study that compared zanabrutinib vs ibrutinib in patients with relapsing CLL or CLL resistant to treatment. In that study, 21.3% of patients on zanubrutinib developed a cardiac event vs 29.6% of patients in the ibrutinib arm (RAR: 8.30 [1.62 to 14.98]).19
To the best of our knowledge, this is the first study to compare the use of zanubrutinib vs ibrutinib as first-line treatment in CLL. There are several network metaanalyses comparing iBTK alone or in combination with venetoclax as add-on therapy to obinutuzumab.
Molica et al. documented no statistically significant differences in PFS between acalabrutinib vs ibrutinib in combination with obinutuzumab across subgroups of patients with different levels of cytogenetic risk.20 In the same vein, Davids et al. found no statistically significant differences in PFS and OS between acalabrutinib and ibrutinib.21 These findings confirm the results of our MAIC analysis of zanubrutinib vs ibrutinib.
A limitation of this study is that acalabrutinib was not included in our MAIC, since it would have required more intermediate comparisons. Another limitation was the impossibility to differentiate patients with a high cytogenetic risk from those with a very high cytogenetic risk; the reason was that results for carriers of different deletions (del17p and del11q), TP53 mutations, and IgHV functional status were not reported separately in the A041202 RCT (Table 1). Additionally, the ESMO-MCBS scale is only available for solid tumors. The ESMO scale for onco-hematological processes is still under development.
In conclusion, assuming the associated uncertainty, the results of this MAIC analysis reveal that zanubrutinib exerts similar clinical benefits in terms of efficacy than ibrutinib. However, differences in safety prevent them from being considered therapeutically equivalent drugs.
Ethical considerationsThis study was conducted in accordance with international ethical principles for the reporting and publication of biomedical research information.
FundingNone.
Conflict of interestThe authors declare no conflict of interest (find attached the «International Committee of Medical Journal Editors» statement form signed by all authors).