The objective of this review is to gather the available evidence on the different drugs used in immune-mediated inflammatory diseases in pregnancy, lactation, their influence on female and male fertility, advice on discontinuation before conception and to help in routine clinical practice for better patient advice on family planning.
MethodsA bibliographic search was carried out, where published articles (review studies, observational studies and case series) in English or Spanish until April 2020 that analyzed the management of pregnancy, lactation and/or fertility in patients on treatment in immune-mediated diseases were selected.
ResultsA total of 95 references were selected and the information on each drug was synthesized in tables. Drugs contraindicated in pregnancy are topical retinoids, pimecrolimus, cyclooxygenase 2 inhibitors, methotrexate, mycophenolate mofetil, leflunomide, acitretin, and thiopurines. The lack of data advises against the use of apremilast, tofacitinib, baricitinib, anakinra, abatacept, tocilizumab and the new biologicals. Topical salicylates, paracetamol, ultraviolet therapy and hydroxychloroquine treatment are safe, and anti-TNF biological therapy are considered low risk, with certolizumab being the drug of choice throughout pregnancy and lactation.
Most are compatible with paternal exposure except for sulfasalazine, mycophenolate and leflunomide, for which suspension of treatment prior to conception is recommended, and cyclosporine with dose requirements of less than 2 mg/kg/day.
ConclusionsIn this context of chronic treatments with teratogenic potential, it is necessary to highlight the importance of pregnancy planning to select the safest drug. Given the quality of the available data, it is still necessary to continuously update the information, as well as to promote observational studies of cohorts of pregnant patients and men of childbearing age, including prospective studies, in order to generate more scientific evidence.
El objetivo de esta revisión es reunir la evidencia disponible de los diferentes medicamentos utilizados en las enfermedades inflamatorias inmunomediadas en la gestación, lactancia, su influencia en la fertilidad femenina y masculina, consejos sobre suspensión antes de la concepción y servir de ayuda en la práctica clínica habitual para un mejor consejo al paciente en la planificación familiar.
MétodoSe realizó una búsqueda bibliográfica, donde se seleccionaron los artículos publicados (estudios de revisión, observacionales y series de casos) en lengua inglesa o española hasta abril de 2020 que analizaban el manejo del embarazo, lactancia y/o fertilidad en pacientes con tratamientos utilizados en las enfermedades inflamatorias inmunomediadas de dermatología, reumatología y digestivo.
ResultadosSe seleccionaron un total de 95 referencias y se sintetizó la información de cada medicamento en tablas. Los fármacos contraindicados en el embarazo son los retinoides tópicos, pimecrolimus, inhibidores de la ciclooxigenasa 2, metotrexato, micofenolato de mofetilo, leflunomida, acitretina y tiopurinas. La falta de datos desaconseja el uso de apremilast, tofacitinib, baricitinib, anakinra, abatacept, tocilizumab y los nuevos biológicos. Mientras que son seguros los salicilatos y emolientes tópicos, el paracetamol, la terapia ultravioleta, la hidroxicloroquina y en la terapia biológica los anti-TNF se consideran de bajo riesgo, siendo el certolizumab el de elección durante todo el embarazo y lactancia. La mayoría son compatibles con la exposición paterna, excepto algunos como la sulfasalacina, micofenolato y leflunomida que se recomienda la suspensión de tratamiento previa a la concepción, y la ciclosporina con requerimientos de dosis inferiores a 2 mg/kg/día.
ConclusionesEn este contexto de tratamientos crónicos con potencial teratogénico, es necesario visibilizar la importancia de la planificación gestacional para seleccionar el fármaco más seguro.
Ante la calidad de los datos disponibles, sigue siendo necesaria la continua actualización de la información, así como el promover estudios observacionales de cohortes de pacientes embarazadas, lactantes y hombres en edad fértil, incluso realizar estudios prospectivos, con el fin de generar mayor evidencia científica.
Immune-mediated inflammatory diseases (IMIDs) are a group of diseases that result from chronic systemic inflammation caused by alterations of the immune system and can affect various organs. This group includes a wide range of diseases, such as rheumatoid arthritis (RA), Crohn's disease (CD), ulcerative colitis, psoriasis (PSO), ankylosing spondylitis (AS), psoriatic arthropathy, hidradenitis suppurativa, lupus, and uveitis.
Although the prevalence of IMIDs remains uncertain, a cross-sectional epidemiological study conducted in Spain in 2017 found that 6.39% of patients had been diagnosed with a relevant IMID. The most prevalent ones were PSO (2.69%) and RA (1.07%)1. They are more common in women and in those of fertile age. A study published in 2017 suggested that active treatment and good control of IMIDs restricted patients' desire for children2. Currently, pregnancy, breastfeeding, and fertility can be discussed with patients, thanks to the available therapeutic armamentarium and studies in clinical practice.
The aim of this systematic review was to collect and summarize the available evidence on various IMID drugs, their effect on pregnancy and breastfeeding, and their effect on female and male fertility. We also provide recommendations regarding the treatment modification before conception.
MethodA bibliographicresearch using Medical Subject Heading (MeSH) terms was conducted to collect information on the management of medications for IMIDs in the setting of conception, pregnancy, and breastfeeding. The following databases were searched: Medline (via Pubmed), Web of Science, and the Cochrane Library.
Articles according to the following inclusion criteria were selected: they were published in English or Spanish in or before 2020; they analyzed pregnancy, breastfeeding, or fertility management for patients in treatment for dermatology, rheumatology, and digestive IMIDs. The selection mainly included review and observational studies, but also included case series. In cases of redundant information, higher quality articles and those that included more cases were selected.
The names of the IMID drugs were combined with following terms: “pregnancy”, “therapy during pregnancy”, “fertility”, “breastfeeding”, “male exposition”, “dermatology”, “rheumatology”, and “digestive”. The search was restricted to autoimmune diseases. The review of the selected articles was performed independently by two of the authors and any disparities were resolved by a third reviewer. The title and abstract of each article was first assessed and if it met the selection criteria, the full text was reviewed.
ResultsIn total, the initial search identified 6013 references. Of these, we selected 32 articles that met the study objectives. After reviewing the articles and their references, a further 93 references were added, thus completing the search. These were used to synthesize the available information and summarize it in the tables.
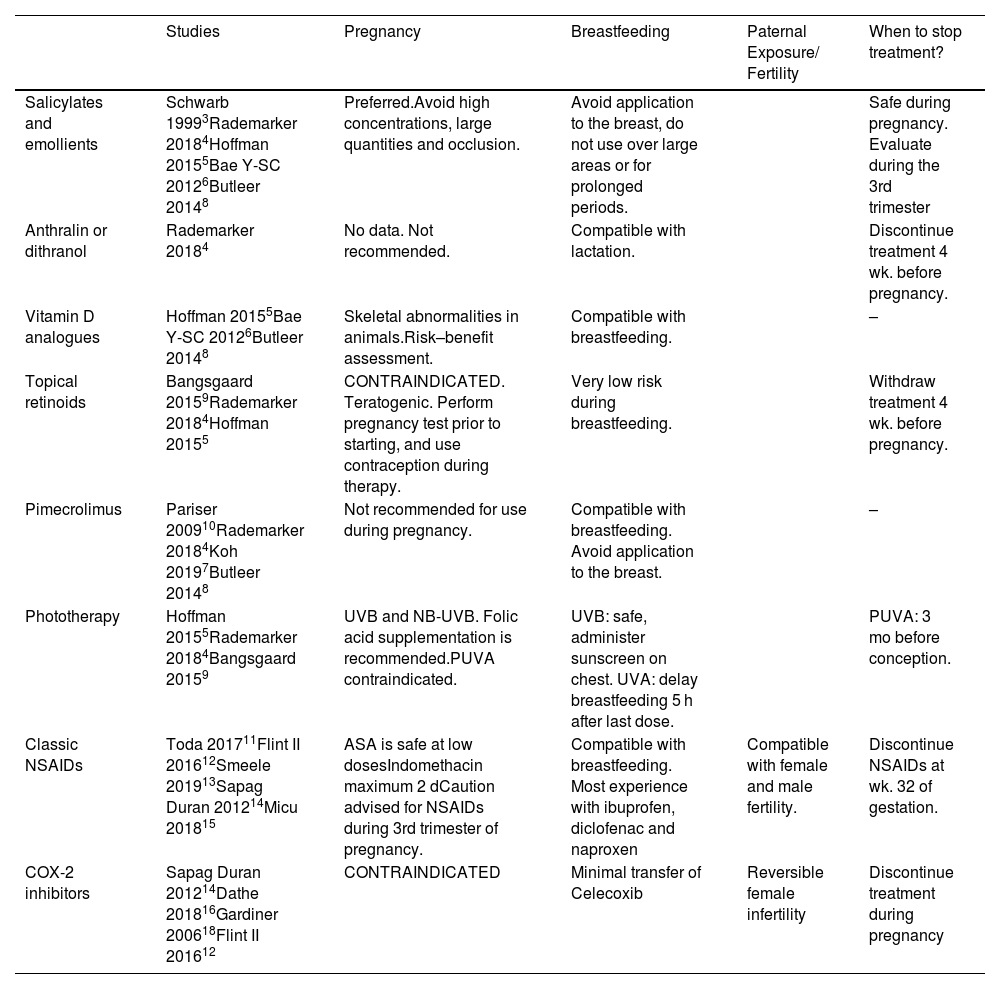
Topical and anti-inflammatory treatments (Table 1)Salicylates and emollientsSalicylates and emollients are drugs of choice for use during pregnancy. Systemic absorption of topical treatment depends on the concentration and duration of application and can be 10% to 25% of the amount applied to the skin3. Therefore, high concentrations (>3%), large quantities (>20 g/d) or use under occlusion should be avoided to prevent penetration4–6.
Topical and anti-inflammatory treatments.
| Studies | Pregnancy | Breastfeeding | Paternal Exposure/ Fertility | When to stop treatment? | |
|---|---|---|---|---|---|
| Salicylates and emollients | Schwarb 19993Rademarker 20184Hoffman 20155Bae Y-SC 20126Butleer 20148 | Preferred.Avoid high concentrations, large quantities and occlusion. | Avoid application to the breast, do not use over large areas or for prolonged periods. | Safe during pregnancy. Evaluate during the 3rd trimester | |
| Anthralin or dithranol | Rademarker 20184 | No data. Not recommended. | Compatible with lactation. | Discontinue treatment 4 wk. before pregnancy. | |
| Vitamin D analogues | Hoffman 20155Bae Y-SC 20126Butleer 20148 | Skeletal abnormalities in animals.Risk–benefit assessment. | Compatible with breastfeeding. | – | |
| Topical retinoids | Bangsgaard 20159Rademarker 20184Hoffman 20155 | CONTRAINDICATED. Teratogenic. Perform pregnancy test prior to starting, and use contraception during therapy. | Very low risk during breastfeeding. | Withdraw treatment 4 wk. before pregnancy. | |
| Pimecrolimus | Pariser 200910Rademarker 20184Koh 20197Butleer 20148 | Not recommended for use during pregnancy. | Compatible with breastfeeding. Avoid application to the breast. | – | |
| Phototherapy | Hoffman 20155Rademarker 20184Bangsgaard 20159 | UVB and NB-UVB. Folic acid supplementation is recommended.PUVA contraindicated. | UVB: safe, administer sunscreen on chest. UVA: delay breastfeeding 5 h after last dose. | PUVA: 3 mo before conception. | |
| Classic NSAIDs | Toda 201711Flint II 201612Smeele 201913Sapag Duran 201214Micu 201815 | ASA is safe at low dosesIndomethacin maximum 2 dCaution advised for NSAIDs during 3rd trimester of pregnancy. | Compatible with breastfeeding. Most experience with ibuprofen, diclofenac and naproxen | Compatible with female and male fertility. | Discontinue NSAIDs at wk. 32 of gestation. |
| COX-2 inhibitors | Sapag Duran 201214Dathe 201816Gardiner 200618Flint II 201612 | CONTRAINDICATED | Minimal transfer of Celecoxib | Reversible female infertility | Discontinue treatment during pregnancy |
During breastfeeding, the application of topical treatments to the breast, large areas of skin, or for prolonged periods should be avoided in order to prevent direct absorption by infants, although significant amounts are unlikely to be found in breast milk7,8.
Topical retinoidsThe retinoids adapalene, tazarotene, and tretinoin are potentially teratogenic during pregnancy9. Although at standard doses teratogenic, mutagenic, or carcinogenic effects have not been observed, teratogenicity may occur if applied to more than 20% of the body surface area4,5.
During breastfeeding, the transcutaneous absorption of tazarotene is very low and thus it appears to be a low-risk treatment.
PimecrolimusDue to its larger molecular size, topical pimecrolimus has poor systemic absorption. Systemic accumulation is negligible after repeated applications, application to large body surface area (up to 69%), or after long-term use10. However, its use is not recommended during pregnancy7,8.
PhototherapyUltra-violet B (UVB) therapy and narrowband UVB (NB-UVB) therapy appear to be safe during pregnancy. However, maternal folate depletion may occur and folic acid supplementation is recommended5. A study that included 107 patients under psoralen+UVA (PUVA) therapy reported cases of premature delivery and foetal abnormalities. The FDA has classified this drug within pregnancy category C. Oral psoralen is administered to patients to increase skin reactivity to UVA rays. There is a theoretical risk of teratogenic and mutagenic effects, which are due to DNA synthesis and the inhibition of cell division. It is recommended to avoid it during pregnancy4,5,9.
Paracetamol and classic non-steroidal anti-inflammatory drugsDuring pregnancy, paracetamol is the safest analgesic and antipyretic drug, but also during periconception and throughout pregnancy. Experimental studies in animals and prospective cohort studies in humans have shown that there are no increases in malformations at therapeutic doses. There is evidence suggesting a positive, although weak, association between prenatal paracetamol use and allergies and asthma in children, adolescents, and adults. This finding is probably related to a decrease in glutathione levels in the lungs, which causes oxidative damage and inflammation. Therefore, it is recommended to use intermittently at the lowest effective dose and for the shortest time possible11,12.
During the first trimester of pregnancy, the use of classic non-steroidal anti-inflammatory drugs (NSAIDs) appears to be free of the risk of teratogenicity or abortion. However, in the third trimester, they can cause premature closure of the ductus arteriosus, pulmonary hypertension, neonatal anuria, anemia, increased risk of pre- and postpartum hemorrhage, and oligohydramnios. They may also increase the risk of intracranial hemorrhage in preterm infants or low-weight neonates by causing decreased platelet aggregation in fetus.
High doses of acetylsalicylic acid (>3 g) inhibit uterine contractility and prolong gestation, whereas during pregnancy the use of low doses as an antiplatelet agent has been shown to be safe12.
Overall, NSAIDs can be used safely during breastfeeding, although some risk of jaundice and kernicterus has been described7,12. Ibuprofen, diclofenac, and naproxen are the most commonly used NSAIDs. Dose-dependent sperm abnormalities have been described in fathers under treatment with NSAIDS. In women, NSAIDs inhibit the production of prostaglandins, which play a role in ovulation and blastocyst implantation13–15.
COX-2 inhibitorsCyclooxygenase 2 (COX-2) inhibitors are associated with an increased risk of premature closure of the ductus arteriosus, especially during the third trimester of pregnancy. Therefore, their use is contraindicated in pregnancy14.
An observational study compared the effect of COX-2 inhibitors in 174 women treated with them during the first trimester of pregnancy and 521 women without COX-2 exposure. No significant differences were found in spontaneous abortions or congenital abnormalities, although more elective abortions were reported in the former group16.
In a cohort of pregnant women in Quebec, the risk of prematurity was 2.46-fold higher in those who were exposed to COX-2 inhibitors in the 3 months prior to delivery than in those who were not exposed to them. With celecoxib, the risk was 3.41-fold higher, suggesting that the use of celecoxib in advanced pregnancy may increase the risk of prematurity17.
Some celecoxib studies have shown that there is a minimal transfer of the drug into breast milk (<0.3%). Therefore, its use is considered to be safe during breastfeeding18, although some guidelines have contraindicated its use12.
Data on male fertility and COX-2 inhibitors are limited and make reference to data on NSAIDs, as described above. In women, the use of COX-2 inhibitors has been associated with reversible infertility due to alterations in ovarian follicle rupture. This effect also occurs with standard NSAIDs. Women trying to conceive are recommended to avoid using them12.
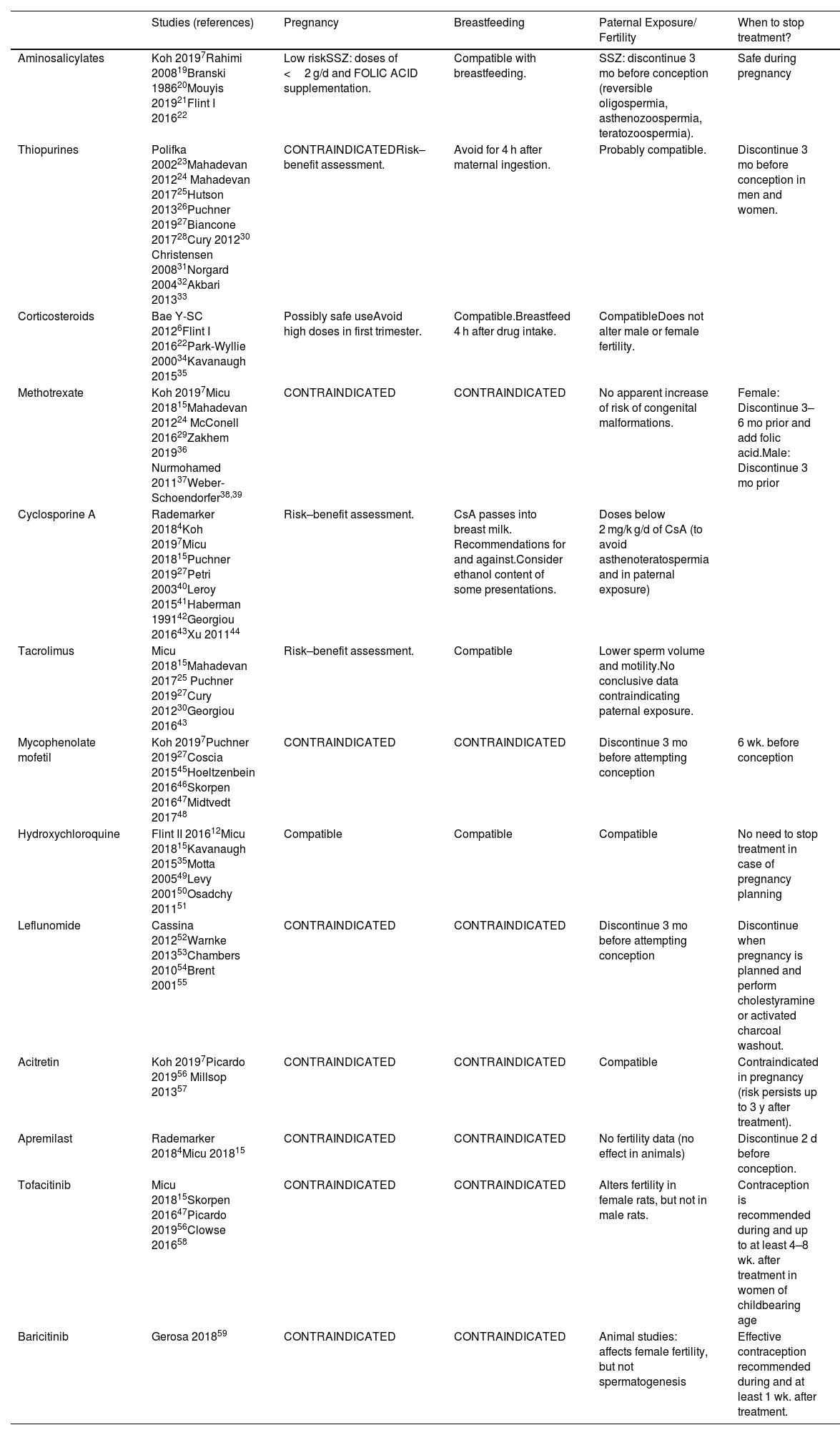
Immunomodulators (Table 2)AminosalicylatesNo adverse effects have been described for the aminosalicylates mesalazine and sulfasalazine (SSZ), and are considered to be of low risk during pregnancy. SSZ has antifolic effects. Thus, folic acid supplements are recommended during the prenatal period and throughout pregnancy and do not exceed doses of 2 g/day. It can be used during breastfeeding because the amount excreted in breast milk is very small. Nevertheless, caution is recommended because cases have been described of blood in faeces20, and diarrhea in infants and jaundice in the newborn7,19.
Immunomodulators.
| Studies (references) | Pregnancy | Breastfeeding | Paternal Exposure/ Fertility | When to stop treatment? | |
|---|---|---|---|---|---|
| Aminosalicylates | Koh 20197Rahimi 200819Branski 198620Mouyis 201921Flint I 201622 | Low riskSSZ: doses of <2 g/d and FOLIC ACID supplementation. | Compatible with breastfeeding. | SSZ: discontinue 3 mo before conception (reversible oligospermia, asthenozoospermia, teratozoospermia). | Safe during pregnancy |
| Thiopurines | Polifka 200223Mahadevan 201224 Mahadevan 201725Hutson 201326Puchner 201927Biancone 201728Cury 201230 Christensen 200831Norgard 200432Akbari 201333 | CONTRAINDICATEDRisk–benefit assessment. | Avoid for 4 h after maternal ingestion. | Probably compatible. | Discontinue 3 mo before conception in men and women. |
| Corticosteroids | Bae Y-SC 20126Flint I 201622Park-Wyllie 200034Kavanaugh 201535 | Possibly safe useAvoid high doses in first trimester. | Compatible.Breastfeed 4 h after drug intake. | CompatibleDoes not alter male or female fertility. | |
| Methotrexate | Koh 20197Micu 201815Mahadevan 201224 McConell 201629Zakhem 201936 Nurmohamed 201137Weber-Schoendorfer38,39 | CONTRAINDICATED | CONTRAINDICATED | No apparent increase of risk of congenital malformations. | Female: Discontinue 3–6 mo prior and add folic acid.Male: Discontinue 3 mo prior |
| Cyclosporine A | Rademarker 20184Koh 20197Micu 201815Puchner 201927Petri 200340Leroy 201541Haberman 199142Georgiou 201643Xu 201144 | Risk–benefit assessment. | CsA passes into breast milk. Recommendations for and against.Consider ethanol content of some presentations. | Doses below 2 mg/k g/d of CsA (to avoid asthenoteratospermia and in paternal exposure) | |
| Tacrolimus | Micu 201815Mahadevan 201725 Puchner 201927Cury 201230Georgiou 201643 | Risk–benefit assessment. | Compatible | Lower sperm volume and motility.No conclusive data contraindicating paternal exposure. | |
| Mycophenolate mofetil | Koh 20197Puchner 201927Coscia 201545Hoeltzenbein 201646Skorpen 201647Midtvedt 201748 | CONTRAINDICATED | CONTRAINDICATED | Discontinue 3 mo before attempting conception | 6 wk. before conception |
| Hydroxychloroquine | Flint II 201612Micu 201815Kavanaugh 201535Motta 200549Levy 200150Osadchy 201151 | Compatible | Compatible | Compatible | No need to stop treatment in case of pregnancy planning |
| Leflunomide | Cassina 201252Warnke 201353Chambers 201054Brent 200155 | CONTRAINDICATED | CONTRAINDICATED | Discontinue 3 mo before attempting conception | Discontinue when pregnancy is planned and perform cholestyramine or activated charcoal washout. |
| Acitretin | Koh 20197Picardo 201956 Millsop 201357 | CONTRAINDICATED | CONTRAINDICATED | Compatible | Contraindicated in pregnancy (risk persists up to 3 y after treatment). |
| Apremilast | Rademarker 20184Micu 201815 | CONTRAINDICATED | CONTRAINDICATED | No fertility data (no effect in animals) | Discontinue 2 d before conception. |
| Tofacitinib | Micu 201815Skorpen 201647Picardo 201956Clowse 201658 | CONTRAINDICATED | CONTRAINDICATED | Alters fertility in female rats, but not in male rats. | Contraception is recommended during and up to at least 4–8 wk. after treatment in women of childbearing age |
| Baricitinib | Gerosa 201859 | CONTRAINDICATED | CONTRAINDICATED | Animal studies: affects female fertility, but not spermatogenesis | Effective contraception recommended during and at least 1 wk. after treatment. |
Abbreviation: SSZ, sulfasalazine.
Reversible oligospermia, asthenozoospermia, and teratozoospermia have been observed in men treated with SSZ, and thus it should be suspended before conception21,22.
ThiopurinesMaternal treatment with azathioprine (AZA) or mercaptopurine (6-MP) during pregnancy is clearly teratogenic in animals at standard doses, but there is limited data on their effects in humans treatment with these drugs may interfere with DNA replication in germ cells by inhibiting nucleic acid synthesis23.
The Pregnancy Inflammatory bowel disease And Neonatal Outcomes (PIANO) registry investigated the effect of exposure to thiopurines and anti-tumor necrosis factor (TNF) in more than 1000 women from conception to delivery. No association was found between thiopurinehiopurine or anti-TNF use and congenital abnormalities or pregnancy complications. However, there was a significant increase in infections at 9 to 12 months of age among the 107 infants exposed to thiopurine andanti-TNF (RR = 1.50, 95% CI 1.08 to 2.09)24. Due to the potential for late infancy infections, the discontinuation of thiopurine after conception could be considered25.
The risk of congenital malformations was higher in women with irritable bowel syndrome (IBD) on thiopurine treatment than in healthy women (RR = 1.45; 95% CI 1.07 to 1.96). However, no increase was seen in comparison to disease controls (RR = 1.37; 95% CI 0.92 to 2.05).
Although most of the current literature does not recommend the administration of thiopurines to pregnant patients7,25,28–30, these results provide support for their use in patients at high risk of relapse27,28.
Mercaptopurine has been detected in the colostrum and breast milk of women receiving AZA treatment. It is recommended to wait 4 h after a dose before breastfeeding, as the peak of 6-MP in milk occurs within the first 4 h after ingestion31.
Although cases of congenital abnormalities have been reported in parents treated with AZA or 6-MP32, Akbari et al.33 did not find significant associations between paternal exposure to thiopurines at the time of conception and congenital abnormalities (combined OR: 1.87; 95% CI 0.67 to 5.25).
CorticosteroidsFetus are at low risk from hydrocortisone, cortisone, prednisolone, and methylprednisolone because they are inactivated in the placenta. Although prednisone does not present teratogenic risk to humans at therapeutic doses, it is recommended to avoid high doses (1–2 mg/kg) in the first trimester due to the risk of cleft palate34. Doses of more than 20 mg/d can cause adrenal hypoplasia in fetus with transient adrenal insufficiency after delivery. Associations have been found between doses of prednisone of more than 5–10 mg/d and an increased risk of gestational diabetes, hypertension, oedema, premature membrane rupture, and osteoporosis. Chronic use causes suppression of adrenocorticotropic hormone (ACTH) production.
Corticosteroids can be administered during breastfeeding, but it is recommended to wait 4 h after a dose before breastfeeding to minimize infant exposure, and especially if the dose is more than 20 mg prednisone/day7,35.
Studies have shown that paternal exposure to prednisolone does not lead to increased rates of malformations15,29,35,36.
MethotrexateMethotrexate (MTX) has been classified by the FDA within pregnancy category X. It is contraindicated during pregnancy because of its teratogenic effect in humans25,29, especially in the first trimester of pregnancy, and even at doses of less than 30 mg/wk. In fact, its embryolethal effect is used in the treatment of ectopic pregnancy37. A prospective multicentre cohort study of 324 pregnant women exposed to MTX found that the incidence of spontaneous abortion and the risk of congenital defects were higher in this group than in the control group. No evidence of increased risk was found in the 136 pregnant women exposed to MTX before pregnancy38,39. It is excreted in breast milk and, because of its long half-life, it accumulates in the tissues of infants. Thus, its use in breastfeeding is contraindicated7.
Cyclosporine A and TacrolimusCyclosporine A (CsA) crosses the placental barrier and can reach fetal blood concentrations of up to 50% of the concentration in maternal blood40. The FDA has classified it within pregnancy category X. However, no teratogenic effects have been reported in humans.
There is no evidence of its negative impact on male and female fertility in patients treated with CsA4,15,41,42. Differences in sperm motility and shape were found in three cohorts of 19, 26, and 212 men who were treated with CsA. These differences were associated with a higher dose of the drug. They also showed normal spermatograms at 2 years after renal transplantation. Thus, it is recommended to wait for 2 years before conception15,43,44. There is no evidence of congenital malformations in children born to parents exposed to CsA44. Thus, doses of less than 2 mg/kg/d CsA do not seem to have a negative effect on male fertility, nor does paternal exposure lead to fetal harm15,43,44.
Regarding tacrolimus, there is no evidence that the risk of its use during pregnancy is higher than the risk of using other immunosuppressants.However, cases of spontaneous abortion have been reported, and so other safer alternatives may be considered25,27,30.
Mycophenolate MofetilMycophenolic acid (MPA) is a teratogenic agent and should not be used during pregnancy45. A prospective European multicentre cohort study of 57 pregnant women who had been exposed to mycophenolate mofetil (MMF) found a 45% cumulative incidence of spontaneous abortion and a 26% risk of severe congenital malformations46. It is recommended that treatment should be discontinued 6 weeks prior to conception7,27,47.
It is contraindicated during the breastfeeding period because of the potential risk of serious adverse reactions to MMF in nursing infants7,27,47.
Three studies on pregnancy after parental exposure to MMF derivatives found that the risk of congenital malformations in the children was no higher than in controls and the general population48. Nevertheless, it is recommended that treatment be discontinued 3 months prior to attempting conception7.
HydroxychloroquineHydroxychloroquine (HCQ) remains the antimalarial drug of choice in women with rheumatic disease who wish to conceive22. A prospective observational study found no association between its use and congenital malformations or neonatal infections in 40 infants born to mothers affected by rheumatic disease under treatment with HCQ. Preterm delivery was identified as the main complication (20.5%), which may be related to the rheumatic disease49.
The results of a double-blind placebo-controlled HCQ study found no congenital abnormalities or neuroophthalmological and auditory abnormalities in infants of 1.5 to 3 years of age50. No evidence of fetal ocular toxicity was found by a systematic review of 12 studies that investigated 588 live births to mothers treated with chloroquine or HCQ during pregnancy51.
Hydroxychloroquine crosses the placenta and its concentration in umbilical cord blood is approximately equal to the maternal concentration. The excretion of HCQ in breast milk is very low (<0.2 mg/kg/day), and so it is considered compatible with breastfeeding22.
LeflunomideLeflunomide and its active metabolite, teriflunomide, cause serious birth defects. Cases have been reported of malformations in children born to mothers exposed to leflunomide during pregnancy52.
In case of exposure, the elimination of teriflunomide can be accelerated by the administration of cholestyramine or activated charcoal35,41,53. A study on women with RA who became pregnant during leflunomide treatment and were treated with cholestyramine found that the percentage of malformations was comparable to the 3% to 4% found in the general population54,55.
Although it remains unknown whether it is excreted in human breast milk, its use is contraindicated during breastfeeding due to the high risk of adverse events in infants.
AcitretinAcitretin is teratogenic and embryotoxic in animals, and thus its use is contraindicated in pregnancy and breastfeeding7,56. It does not appear to affect male fertility57. Post-authorization studies of the drug have found no evidence of reproductive risk56.
ApremilastIts use during pregnancy and breastfeeding is contraindicated. Experimental animal studies have found dose-relatedembryofetal loss, reduced birth weight, and delayed ossification in mice at doses higher than the currently recommended maximum human dose. These effects have not been observed in animals exposed to 1.3 times the clinical dose4.
Janus-kinase inhibitors:Tofacitinib and baricitinibData on their use in humans are sparse and unclear due to frequent concomitant use with MTX. Tofacitinib has been shown to be teratogenic in rats and rabbits, and affects parturition and peri- and postnatal development.
ofacitinib clinical safety databases, post-marketing studies in RA, and adverse event reports showed that out of 47 pregnancies, one baby was born with a congenital anomaly. There were also seven spontaneous abortions, of which three occurred when used in combination with MTX58.
It is not known whether tofacitinib is excreted in breast milk. A risk for infants cannot be excluded because the low molecular weight of the tofacitinib molecule could allow its passage into breast milk. Its use during breastfeeding is contraindicated as a precautionary measure47,56.
There are insufficient data on the use of baricitinib in pregnant women and whether it is excreted in breast milk. Animal studies have shown that it is toxic and excreted into breast milk. It is teratogenic in rats and rabbits because it affects in utero bone development at high doses. A risk to fetus or newborns cannot be excluded, and thus o it is generally contraindicated during pregnancy and breastfeeding59.
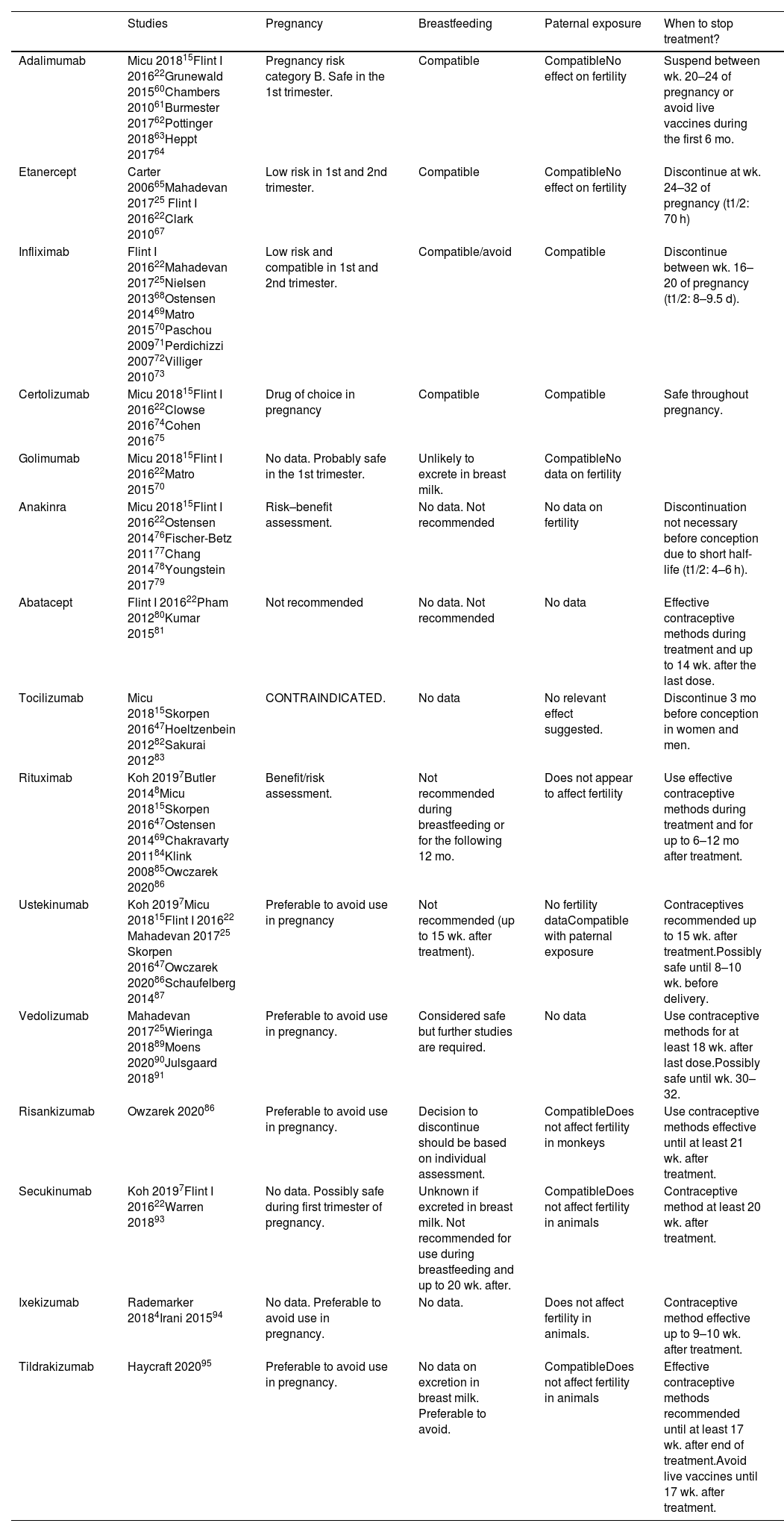
Biologic therapy (Table 3)Anti-TNFs were the first biologics to be developed. Due to greater experience in their use, they are preferred to the use of new antibodies for therapeutic targets such as interleukin (IL) 12, IL-17 or IL-23.
Biologic therapies.
| Studies | Pregnancy | Breastfeeding | Paternal exposure | When to stop treatment? | |
|---|---|---|---|---|---|
| Adalimumab | Micu 201815Flint I 201622Grunewald 201560Chambers 201061Burmester 201762Pottinger 201863Heppt 201764 | Pregnancy risk category B. Safe in the 1st trimester. | Compatible | CompatibleNo effect on fertility | Suspend between wk. 20–24 of pregnancy or avoid live vaccines during the first 6 mo. |
| Etanercept | Carter 200665Mahadevan 201725 Flint I 201622Clark 201067 | Low risk in 1st and 2nd trimester. | Compatible | CompatibleNo effect on fertility | Discontinue at wk. 24–32 of pregnancy (t1/2: 70 h) |
| Infliximab | Flint I 201622Mahadevan 201725Nielsen 201368Ostensen 201469Matro 201570Paschou 200971Perdichizzi 200772Villiger 201073 | Low risk and compatible in 1st and 2nd trimester. | Compatible/avoid | Compatible | Discontinue between wk. 16–20 of pregnancy (t1/2: 8–9.5 d). |
| Certolizumab | Micu 201815Flint I 201622Clowse 201674Cohen 201675 | Drug of choice in pregnancy | Compatible | Compatible | Safe throughout pregnancy. |
| Golimumab | Micu 201815Flint I 201622Matro 201570 | No data. Probably safe in the 1st trimester. | Unlikely to excrete in breast milk. | CompatibleNo data on fertility | |
| Anakinra | Micu 201815Flint I 201622Ostensen 201476Fischer-Betz 201177Chang 201478Youngstein 201779 | Risk–benefit assessment. | No data. Not recommended | No data on fertility | Discontinuation not necessary before conception due to short half-life (t1/2: 4–6 h). |
| Abatacept | Flint I 201622Pham 201280Kumar 201581 | Not recommended | No data. Not recommended | No data | Effective contraceptive methods during treatment and up to 14 wk. after the last dose. |
| Tocilizumab | Micu 201815Skorpen 201647Hoeltzenbein 201282Sakurai 201283 | CONTRAINDICATED. | No data | No relevant effect suggested. | Discontinue 3 mo before conception in women and men. |
| Rituximab | Koh 20197Butler 20148Micu 201815Skorpen 201647Ostensen 201469Chakravarty 201184Klink 200885Owczarek 202086 | Benefit/risk assessment. | Not recommended during breastfeeding or for the following 12 mo. | Does not appear to affect fertility | Use effective contraceptive methods during treatment and for up to 6–12 mo after treatment. |
| Ustekinumab | Koh 20197Micu 201815Flint I 201622 Mahadevan 201725 Skorpen 201647Owczarek 202086Schaufelberg 201487 | Preferable to avoid use in pregnancy | Not recommended (up to 15 wk. after treatment). | No fertility dataCompatible with paternal exposure | Contraceptives recommended up to 15 wk. after treatment.Possibly safe until 8–10 wk. before delivery. |
| Vedolizumab | Mahadevan 201725Wieringa 201889Moens 202090Julsgaard 201891 | Preferable to avoid use in pregnancy. | Considered safe but further studies are required. | No data | Use contraceptive methods for at least 18 wk. after last dose.Possibly safe until wk. 30–32. |
| Risankizumab | Owzarek 202086 | Preferable to avoid use in pregnancy. | Decision to discontinue should be based on individual assessment. | CompatibleDoes not affect fertility in monkeys | Use contraceptive methods effective until at least 21 wk. after treatment. |
| Secukinumab | Koh 20197Flint I 201622Warren 201893 | No data. Possibly safe during first trimester of pregnancy. | Unknown if excreted in breast milk. Not recommended for use during breastfeeding and up to 20 wk. after. | CompatibleDoes not affect fertility in animals | Contraceptive method at least 20 wk. after treatment. |
| Ixekizumab | Rademarker 20184Irani 201594 | No data. Preferable to avoid use in pregnancy. | No data. | Does not affect fertility in animals. | Contraceptive method effective up to 9–10 wk. after treatment. |
| Tildrakizumab | Haycraft 202095 | Preferable to avoid use in pregnancy. | No data on excretion in breast milk. Preferable to avoid. | CompatibleDoes not affect fertility in animals | Effective contraceptive methods recommended until at least 17 wk. after end of treatment.Avoid live vaccines until 17 wk. after treatment. |
Notes: t1/2, half-life.
Like other anti-TNFs, adalimumab is classified within pregnancy category B. A registry of 500 pregnant women found no evidence of embryotoxicity, teratogenicity, and spontaneous abortions60. Subsequent studies on AR and CD found that the relative risk of birth defects and spontaneous abortion was no higher in the adalimumab-exposed group than in controls. However, the risk of preterm delivery was higher in this group than in healthy women, regardless of adalimumab-exposure61,62.
Treatment with anti-TNFs during pregnancy could lead to neonatal immunosuppression and an increased risk of infections. Therefore, vaccination with live vaccines should be avoided during the first 6 months at least63 or adalimumab treatment should be suspended between week 20 and 24 of pregnancy22,47.
It is excreted through breast milk in very low concentrations. This IgG antibody undergoes intestinal proteolysis and has low bioavailability when taken orally. It can be used during breastfeeding, given that no adverse effects have been reported in children8.
A recent study analyzed the semen of 14 patients exposed to the drug and found no evidence of an effect on male fertility or harm to their offspring15 and no relevant or negative effects on fertility64.
EtanerceptSeveral cohort and case control studies including more than 300 pregnant women have shown no evidence of an increased risk of major birth defects47, although a case has been reported of a child born with Vater's disease after etanercept exposition during pregnancy65.
In 138 women exposed to etanercept, there were six spontaneous abortions, two terminations (one for cardiac anomaly and one Turner syndrome) and 130 births with no malformation patterns66.
Etanercept has been reported to be excreted in breast milk after subcutaneous administration. The aforementioned guidelines suggest that it is compatible with breastfeeding22,47. According to a recent review of anti-TNFs, etanercept does not appear to affect male fertility or harm offspring67.
InfliximabInfliximab is an anti-TNF IgG1 monoclonal antibody. A systematic review reported that the PIANO registry found no differences in congenital abnormalities in the offspring of 260 women exposed to infliximab during pregnancy and in those of the control group68. Starting at week 30 of pregnancy, mean infliximab levels reach concentrations of 160% of the mother's and remain detectable for up to 6 months69.
It is excreted in milk and it is recommended that breastfeeding should be avoided for at least 6 months after treatment. The PIANO registry found no risk of infection or growth retardation in children whose mothers were treated with thiopurines or anti-TNFs70. Some guidelines suggest that it is compatible.
A discrete case series found that infliximab does not affect male or female fertility and is neither genotoxic nor embryotoxic71. However, in vitro studies have found evidence of decreased sperm motility and integrity72,73.
CertolizumabCertolizumab is the biologic drug of choice for pregnant women. A registry of 1137 patients found no evidence of teratogenicity or an increased risk of fetal death74. Its elimination half-life is 14 days. However, it does not have an Fc region and does not cross the placenta. Thus, guidelines recommend maintaining treatment throughout pregnancy22,47.
Its transfer from maternal plasma to milk is minimal and can therefore be administered during breastfeeding. According to the results of a clinical trial in 20 healthy men, semen quality and other semen properties were similar under certolizumab or placebo15.
AnakinraAnakinra is an IL 1α and IL 1β inhibitor. Animal studies have shown that it does not have negative effects on fetus despite being detected in amniotic fluid, even at doses 100 times the therapeutic dose76. Previous case series have reported spontaneous abortion and two babies with congenital abnormalities77–79. Due to the lack of data, it is not generally recommended to continue treatment during pregnancy or during breastfeeding.
AbataceptAnimal studies have shown that abatacept crosses the placenta, and can reach fetal concentrations 1.7 to 2.4 times lower than those in maternal serum. Eratogenicity has not been demonstrated in mice at doses of up to 30 times higher than those used in humans80.
A recent study of 151 pregnant women exposed to abatacept reported 86 live births, 40 spontaneous abortions, and 19 elective abortions. In total, 51.3% of the participants had received concomitant MTX.Seven of the babies presented congenital abnormalities and of these, three were exposed to other concomitant teratogenic medications, such as mycophenolate and leflunomide81.
TocilizumabTocilizumab is a monoclonal antibody against the IL-6 receptor. A study found a high rate of miscarriages, but no congenital malformations, in the offspring of women under concomitant treatment with MTX47. Due to a lack of data, the use of tocilizumab is not recommended during pregnancy or breastfeeding82.
Nonclinical data suggest that female fertility is unaffected by treatment with tocilizumab83. Animal studies using doses of up to 10 times higher than those used in humans have found no evidence of abnormalities in male fertility.No congenital malformations were recorded in 22 cases of pregnancies involving parents exposed to Tocilizumab15.
RituximabRituximab is a monoclonal antibody medication that binds specifically to the CD20 antigen of B lymphocytes. B-lymphocyte levels have not been determined in the newborns of rituximab-exposed women, but transient B-cell depletion and lymphocytopaenia have been reported in some infants born to women exposed to rituximab during pregnancy47.
Exposure prior to pregnancy and during the first trimester does not appear to put fetus at excessive risk84, although transient lymphopaenia or neutropaenia have been reported. It suppresses neonatal B-cell development in fetus during the 2nd and 3rd trimester of pregnancy, but B-cell counts recover during the first 6 months of life76. Studies have found an increase in miscarriages, although remains unknown if they are directly related to exposure to the drug or to the disease. No increase has been found in congenital malformations47,84.
It is unknown whether rituximab is excreted in breast milk47. However, maternal IgG is excreted in milk and thus it is recommended that women should not breastfeed their children during treatment with rituximab or during the following 12 months8.
Studies have found that children exposed to maternal rituximab treatment during pregnancy have a normal response to vaccination. However, due to the limited data, caution is advised regarding its administration during pregnancy76,85.
UstekinumabUstekinumab is an IgG1 monoclonal antibody that inhibits cytokines IL-12 and IL-23. Animal studies have shown that it has no adverse effects on pregnancy, embryo/fetal development, parturition, or postnatal development. Nevertheless, there are insufficient data on the use of ustekinumab in pregnant women and thus its use should be avoided7,86. One review referenced pregnancy outcomes for 26 of 29 pregnancies, of which 5 involved spontaneous abortions (19%). This rate is similar to that of the general population87. Other reviews have suggested that ustekinumab may be safe and have recommended its use even up to 8 to 10 weeks before delivery25.
VedolizumabVedolizumab is a monoclonal antibody that targets the a4ß7 integrin, which is mainly expressed on T-helper lymphocytes that are recruited to the intestine
A case series of 24 women treated with vedolizumab reported 12 live births, four spontaneous abortions, five selective abortions, and one congenital anomaly88. Although its half-life is three times that of infliximab (25 days), it was found that levels were lower in newborns than in the 7 mothers who were exposed to treatment during pregnancy89.
The European CONCEIVE study was a retrospective observational case–control study of pregnant women with IBD. It included 79 women exposed to vedolizumab, a control group of 186 women exposed to anti-TNFs, and 184 unexposed women. No significant differences were found in the rate of spontaneous abortion between the vedolizumab and anti-TNF groups, or between the vedolizumab and unexposed groups. The mean gestational age, weight, and prematurity of newborns were similar between groups, even after correcting for disease activity during pregnancy. There were no differences in congenital abnormalities, nor were malignancies detected at 1 year after birth. Further prospective studies may be needed to confirm these data90.
Vedolizumab has been detected in breast milk with a peak of less than 1% of maternal serum concentrations91,92. Thus, although it is considered safe, further studies are required to evaluate its effect on the infant immune system25.
SecukinumabSecukinumab is a fully human IgG1/κ monoclonal antibody that inhibits IL-17A. There is a lack of data on the use of secukinumab in pregnant women. Animal studies have found no evidence of negative effects on pregnancy, embryonic or fetal development, parturition, or postnatal development.
One study analyzed a safety database that included 238 cases of maternal exposure and 54 cases of paternal exposure. Most patients discontinued secukinumab treatment in the first trimester of pregnancy. The miscarriage rate was 10.3% and there were three cases of congenital abnormalities. These outcomes are similar to those found in the general population93.
IxekizumabIxekizumab is an IgG4-type monoclonal antibody that inhibits IL-17A.
Animal studies have found no evidence of negative effects on pregnancy, embryonic or fetal development, parturition, or postnatal development. There are insufficient data on the use of ixekizumab in pregnant women and it is recommended that its use should be avoided4. Given that it is an IgG4 antibody, it is thought that its rate of transport across the placenta is less than that of other monoclonal antibodies with an IgG1 structure94.
It remains unknown whether ixekizumab is excreted in breast milk or absorbed after ingestion. However, as it is a high molecular weight protein, it is thought that it is not absorbed during the first few weeks postpartum4,87.
TildrakizumabTildrakizumab is a humanized IgG1/κ-type monoclonal antibody that inhibits IL-23.
Animal studies have found no evidence of harmful effects on reproduction, but data on pregnant women are limited. Clinical trials with tildrakizumab in women who became pregnant did not find increased rates of spontaneous abortions or congenital abnormalities95.
It remains unknown if tildrakizumab is excreted in breast milk. No data are available on the response to live microorganism vaccines, and thus it is recommended that they are avoided during treatment and for at least 17 weeks thereafter86.
DiscussionThis review provides updated information on medications used during pregnancy, breastfeeding, and fertility for immune-mediated diseases. This information may be used to advise people who wish to conceive and to make the best decisions in cases of accidental pregnancy.
The following teratogenic drugs should be avoided during pregnancy: Retinoids, PUVA therapy, COX inhibitors, thiopurines, MTX, MMF, leflunomide, and acitretin. The lack of data makes it inadvisable to use JAK inhibitors and apremilast. Paracetamol, hydroxychloroquine, and corticosteroids are considered to be relatively safe. In contrast, a recent prospective study (the PIANO registry) has highlighted the relevance of controlling disease activity in patients with IBD with steroid-sparing therapy before and during pregnancy due to the increased risk of preterm delivery and serious infections at 9 months and 12 months after the use of corticosteroids during the second and/or third trimester96.
Anti-TNF drugs are considered to be low risk in the first and second trimester of pregnancy, with certolizumab being the drug of choice during pregnancy and breastfeeding. A recent meta-analysis of patients with IBD found an association between disease activity and an increased incidence of preterm delivery, low birth weight, and small size relative to gestational age. The use of anti-TNFs during pregnancy has been associated with a higher incidence of admission to intensive care and low birth weight97. Due to the limited data available, the remaining biologics should be used with caution and only after individualized risk–benefit assessments.
This study has some relevant limitations associated with the quality of the available data. In general, the studies cited were statistically underpowered, they were unadjusted for confounding factors (e.g. disease activity or the concomitant use of disease-modifying antirheumatic drugs), and did not address the impact of pregnancy on the course of the disease. The studies were also very heterogeneous regarding the degree of exposure to biologics, the definition of adverse outcomes, and the follow-up period. These aspects limit the ability to extrapolate from these findings and thus these data should be interpreted with caution.
Given the absence of robust data on paternal exposure, we recommend avoiding the use of these drugs. However, this does not imply that there are data available that suggests that they should not be used. Fertility counseling should also be included in family planning programs for men who wish to become fathers.
More evidence needs to be collected through regular updates, observational studies of cohorts of pregnant patients, and studies on paternal exposure. Registries of mothers and fathers exposed to drugs, such as the PIANO registry, the BADBIR registry (British Association of Dermatologist Biologics Intervention Register), the European Psoriasis Registry Network (PSONET), and the Psoriasis Longitudinal Assessment and Registry (PSOLAR), are key to obtaining high-quality data98. Similarly, scientific societies99 are also essential in that they establish consensus guidelines and recommendations for good practice based on the available evidence, addressing issues such as the use of contraception to avoid unplanned pregnancy, the provision of prepregnancy counseling to plan pregnancies during periods of stable disease, the use of medication compatible with pregnancy, and the promotion of shared decision making with the patient.
FundingThe study has been carried out without funding.
On behalf of the Anti-Inflammatory Diseases Working Group.