The main objective is to analyse unjustified discrepancies found during the medication reconciliation process in patients admitted to the Haematology Service of our hospital, in addition to the pharmaceutical interventions carried out. As a secondary objective, to detect possible points of the procedure to be perfected with a view to protocolizing the medication reconciliation process in haematological patients that adapts to the conditions of our center.
MethodsCross-sectional observational pilot study carried out in a reference hospital in haematology for a population of 800 000 inhabitants. Adult inpatients admitted to the Haematology Service between August and October 2022 whose medication had been reconciled were included. The main variables were: number and type of unjustified discrepancy, proposed pharmaceutical intervention, and degree of acceptance.
Results36 conciliation processes were analysed, 34 admissions and 2 intrahospital transfer. 58.3% of the patients presented some unjustified discrepancy. 38 unjustified discrepancies were detected, with an acceptance of pharmaceutical interventions of 97.4%. The most common types of discrepancy were medication omission (56.8%) and drug interaction (24.3%). The most frequent pharmaceutical interventions were reintroducing medication (48.6%) and suspending treatment (16.2%). Polypharmacy and receiving chemotherapy treatment multiply by 4 the probability of presenting drug interactions.
ConclusionsThe most common unjustified discrepancies in the medication reconciliation process in hospitalized haematology patients are: medication omission and drug interactions. The reintroduction of medication and suspension of the prescription are the most frequent accepted pharmaceutical interventions. Polypharmacy is related to an increase in unjustified discrepancies. The factors that promote the appearance of interactions are admissions to receive chemotherapy treatment and polypharmacy. The main point of improvement detected is the need to create a circuit that allows conciliation to be carried out on discharge. Medication reconciliation contribute to improving patient safety by reducing medication errors.
El objetivo principal es analizar las discrepancias no justificadas detectadas en el proceso de conciliación de medicación en pacientes ingresados en el servicio de hematología de nuestro hospital, además de las intervenciones farmacéuticas realizadas. Como objetivo secundario, detectar posibles puntos del procedimiento a perfeccionar de cara a la protocolización del proceso de conciliación de medicación en paciente hematológico que se adapte a las condiciones de nuestro centro.
MétodosEstudio piloto observacional transversal realizado en un hospital de referencia en hematología para una población de 800.000 habitantes. Se incluyeron pacientes adultos ingresados en el Servicio de Hematología entre agosto y octubre del 2022 a los que se les concilió la medicación. Las variables principales fueron: número y tipo de discrepancia no justificada, intervención farmacéutica realizada y grado de aceptación.
ResultadosSe analizaron 36 procesos de conciliación, 34 ingresos y 2 traslados intrahospitalarios. El 58,3% de los pacientes presentó alguna discrepancia no justificada. Se detectaron 38 discrepancias no justificadas, con una aceptación de las intervenciones farmacéuticas del 97,4%. Los tipos de discrepancia más habituales fueron omisión de medicación (56,8%) e interacción farmacológica (24,3%). Las intervenciones farmacéuticas más frecuentes fueron reintroducir medicación (48,6%) y suspender tratamiento (16,2%). La polifarmacia y recibir tratamiento quimioterápico multiplican por 4 la probabilidad de presentar interacciones farmacológicas.
ConclusionesLas discrepancias no justificadas más habituales del proceso de conciliación de medicación en pacientes hematológicos ingresados son: omisión de medicación e interacciones farmacológicas. La reintroducción de medicación y suspensión de la prescripción son las intervenciones farmacéuticas aceptadas más frecuentes. La polifarmacia se relaciona con un incremento de discrepancias no justificadas. Los factores que fomentan la aparición de interacciones son los ingresos para recibir tratamiento quimioterápico y la polifarmacia. El principal punto de mejora detectado es la necesidad de crear un circuito que permita llevar a cabo la conciliación al alta. La conciliación de medicación contribuye a mejorar la seguridad del paciente al disminuir los errores de medicación.
Medication errors (MEs) are a leading cause of adverse events.1 Up to 50% of all MEs occur during care transitions due to poor communication of information.2
The Treatment Reconciliation Process (TRP) involves ensuring that patients continue to receive the necessary medications they were taking prior to the transition of care, at the correct dose, frequency, and route of administration, and in a manner that is appropriate to their clinical situation. It is therefore essential to assess the consistency and appropriateness of chronic medications with those prescribed in the hospital. Possible duplications, drug–drug interactions (DDIs), contraindications due to patients' condition, and newly prescribed treatments must be carefully monitored. Any unwarranted discrepancy (UD) is considered a reconciliation error and therefore an ME. Previous studies have found that approximately 50% of patients experience reconciliation errors.3,4
A high proportion of adverse drug events occur in elderly, polymedicated, and/or multimorbid patients.1,5–7 Polymedicated older patients are therefore at greater risk of being prescribed potentially inappropriate medications. Several studies have found that many older patients receiving care from oncology services are polymedicated. A notable number of these patients receive oncological therapies concurrently with other supportive treatments, which increases their overall drug burden and the risk of DDIs and adverse reactions. In addition, patients often take over-the-counter medications, vitamin or mineral supplements, or herbal products that may contain potentially inappropriate substances and/or contribute to DDIs.7–10
Furthermore, a considerable proportion of these older patients are characterized as frail, as cancer exacerbates the physiological changes that are associated with the ageing process. Frailty is defined as a state of vulnerability arising from diminished reserves in multiple organ systems, triggered by disease, inactivity, inadequate nutrition, stress, and/or ageing.10,11 Cancer can alter organ physiology and influence the pharmacokinetic management and pharmacodynamic sensitivity of drugs. In cases of renal or hepatic failure, dose adjustments may be required.10
Oncohaematology patients requiring hospital admission typically exhibit a number of the characteristics previously outlined, which renders them inherently complex patients. Medication reconciliation (Med Rec) is therefore of particular importance to ensure the safety and optimal comprehensive care of this patient group.
The Spanish Institute for the Safe Medication Practice (ISMP) recommends that the TRP should be systematic, structured, and comprehensive and that it should take into account the perspectives of healthcare professionals, patients, relatives, and caregivers. It has been suggested that a rigorous standardized TRP is the best way to minimize MEs during care transitions.12
Clinical pharmacy plays a pivotal role in ensuring patients receive safe and comprehensive care. Clinical pharmacists specializing in oncohaematology, when integrated into the medical team, are among the most appropriate professionals to perform Med Rec in this setting. They are experts in cancer treatment, professionally trained in pharmacology, and experienced in the use of evidence-based medicine.13–15
Currently, our hospital does not have a Med Rec protocol. Moreover, there are virtually no articles on Med Rec in this specific population. We designed a pilot study after the haematology department expressed interest, given the potential of Med Rec to significantly improve the comprehensive treatment of hospitalized haematological patients if integrated into routine practice. The study aim was to analyse the situation prior to the creation and implementation of a Med Rec protocol for haematological patients requiring admission to the haematology department, including the different care transitions and with the participation of the professionals involved: doctors, nurses, and pharmacists. This study also serves as a baseline for future evaluations of the effectiveness of the Med Rec protocol in oncohaematological patients.
The objective was to analyse UDs found during TRPs in patients admitted to the haematology department of our hospital and any associated pharmaceutical interventions (PIs). A secondary objective was to identify potential areas of the TRP that should be addressed by the Med Rec protocol for haematology patients, tailored to the conditions of our hospital.
MethodsDesign and type of studyThis cross-sectional, observational study was conducted in a haematology reference hospital serving a population of approximately 800 000 inhabitants. Inclusion criteria were adult patients admitted to the haematology department between August and October 2022, who had undergone TRPs, and whose data and PIs were recorded on the Med Rec record card used by the pharmacy department (Appendix 1). Patients with multiple admissions during the study period were considered as separate patients, as they underwent a different TRP each time.
The TRP is defined as obtaining a complete and up-to-date list of the patients' medications as appropriate to their clinical situation. Data were collected through interviews with patients, family members, and/or caregivers, and from electronic prescribing programmes. DDIs were analysed using the Lexicomp Drugs Interaction programme,16 and were considered to be clinically relevant if they were classified as D (consider modifying treatment) or X (avoid combination). Polypharmacy was defined as taking 5 or more drugs.6,7
We classified UDs into the following groups: medication omission, different dosage, different frequency, different route, incorrect presentation, duplication, unjustified initiation of medication/prescription, relevant DDIs, allergy, or adverse drug reaction.3
Data sourcesPatients were selected by reviewing Med Rec records for the study period. Study variables were collected through Med Rec records, medical history (SAP), and electronic home and hospital medication prescription (Silicon, eCAP, Shared Medical Record, and Farmis_Oncofarm).
Study variablesDemographic and baseline characteristics were as follows: sex, age, date of admission, date of reconciliation, main haematological diagnosis according to ICD-10 classification at one decimal place,17 drug allergies or intolerances, outpatient treatment prior to admission (including over-the-counter medication, supplements, or phytotherapy products), hospital treatment on admission, DDIs, reason for admission, and chemotherapy treatment schedule.
The main variables were number and type of UD, the proposed PIs, and the degree of acceptance of these variables by the haematology department.
Additional variables were collected: ATC classification,18 including the anatomical group letter and the second-level number of the drugs involved, concomitant diseases, polypharmacy, time of reconciliation, adherence to hospital guidelines, and number of admissions to haematology during the study period.
Data analysisQuantitative variables are presented as means with a 95% confidence interval (95% CI) if normally distributed, or as medians and percentiles (25–75 interquartile range) for asymmetric distributions. The t-test was used for comparisons between 2 means (p<.05). The geometric mean was used to estimate associations between variables. Qualitative variables are expressed as frequencies and percentages. All statistical analyses were performed using Excel.
The study was approved by the Medical Research Ethics Committee of our hospital.
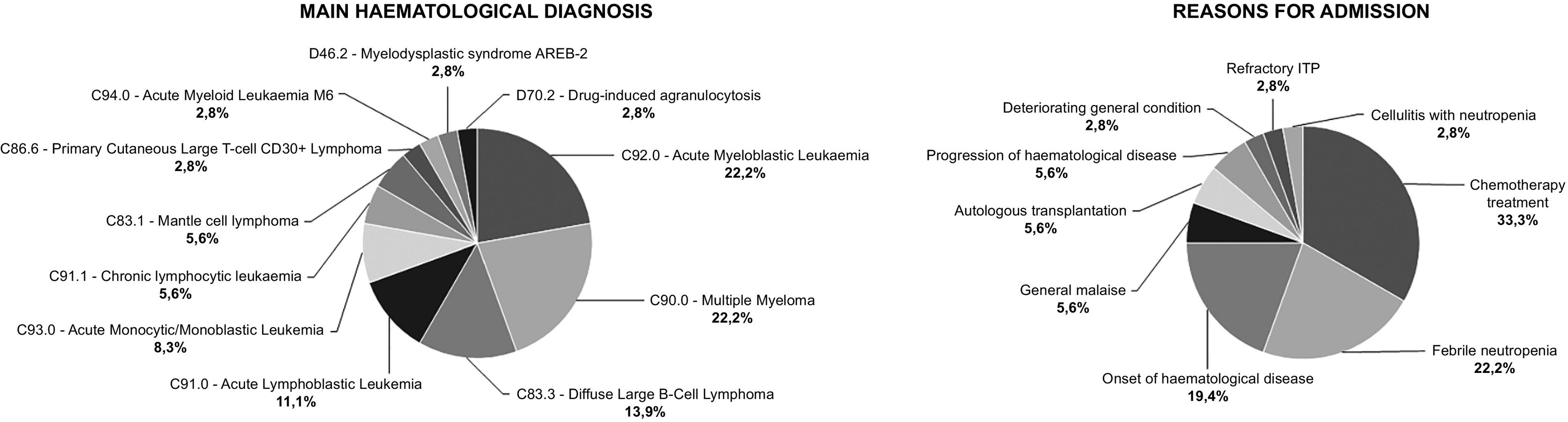
ResultsWe analysed 36 TRPs. Table 1 shows the baseline variables for the study population and Med Recs. Fig. 1 shows the distribution of patients according to the main haematological diagnosis and the reason for admission.
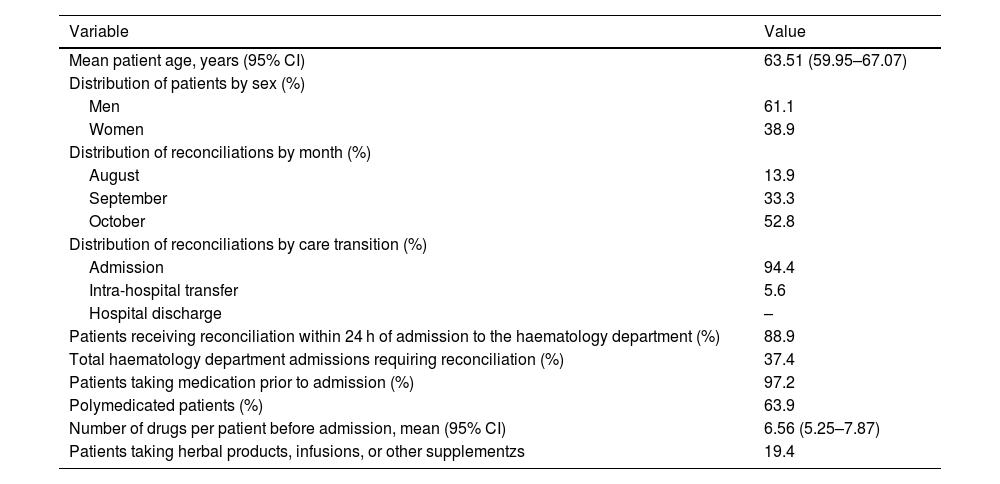
Baseline variables of the reconciliation processes analysed and the study population.
| Variable | Value |
|---|---|
| Mean patient age, years (95% CI) | 63.51 (59.95–67.07) |
| Distribution of patients by sex (%) | |
| Men | 61.1 |
| Women | 38.9 |
| Distribution of reconciliations by month (%) | |
| August | 13.9 |
| September | 33.3 |
| October | 52.8 |
| Distribution of reconciliations by care transition (%) | |
| Admission | 94.4 |
| Intra-hospital transfer | 5.6 |
| Hospital discharge | – |
| Patients receiving reconciliation within 24 h of admission to the haematology department (%) | 88.9 |
| Total haematology department admissions requiring reconciliation (%) | 37.4 |
| Patients taking medication prior to admission (%) | 97.2 |
| Polymedicated patients (%) | 63.9 |
| Number of drugs per patient before admission, mean (95% CI) | 6.56 (5.25–7.87) |
| Patients taking herbal products, infusions, or other supplementzs | 19.4 |
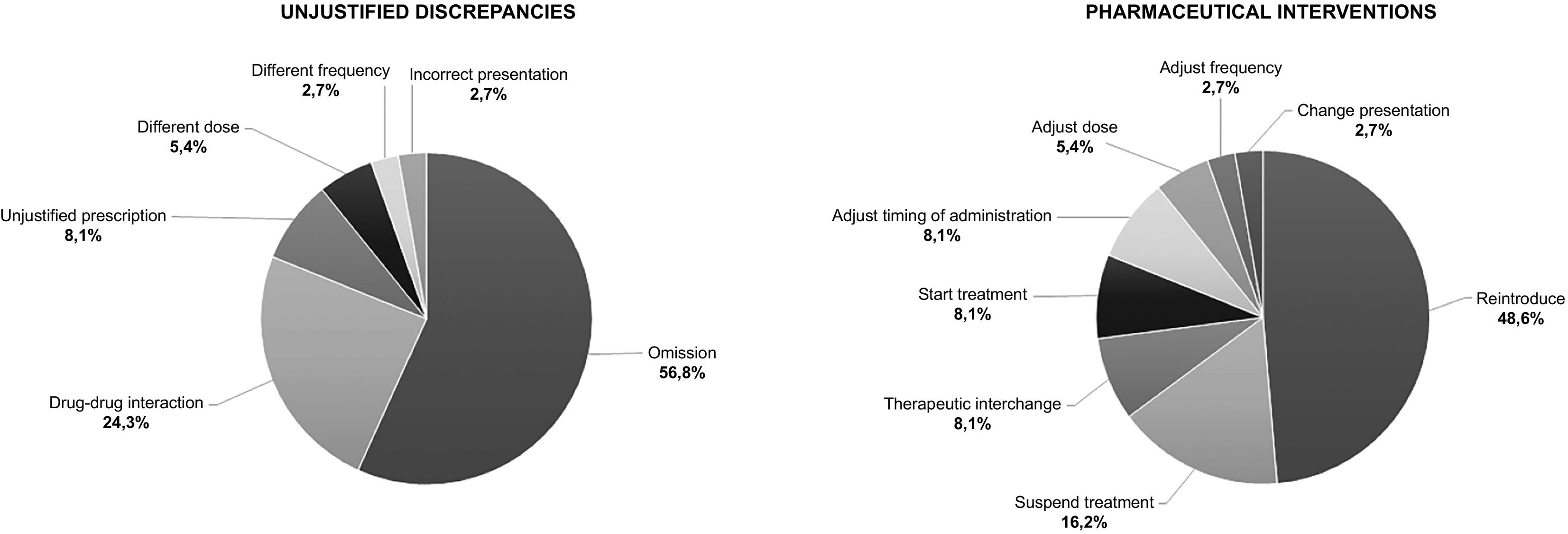
We found UDs in 58.3% of the TRPs (total UDs=38; mean=1.06 UD/patient [95% CI, 0.66–1.45]), and conducted PIs on all UDs. Of these PIs, 97.4% were accepted by the haematology department. Fig. 2 shows the distribution of UDs and PIs by type.
In total, UDs were found in 69.6% of polymedicated patients vs 38.5% of non-polymedicated patients. Thirty-one UDs (83.8% of the total) were found in polymedicated patients. There was a mean of 1.35 UD/patient (95% CI 0.85–1.85) in polymedicated patients and 0.46 UD/patient (95% CI, 0.10–0.82) in non-polymedicated patients. Thus, the likelihood of UDs was 2.92 times higher in polymedicated patients compared to non-polymedicated patients. The difference between the 2 means was significant (p=.02).
Category D (consider modifying treatment) accounted for 66.7% of UDs due to DDIs requiring PI, and 33.3% were Category X (avoid combination). We found 0.35 DDIs/polymedicated patient (95% CI, 0.15–0.55) compared to 0.08 DDIs/non-polymedicated patient (95% CI, −0.07–0.23). A total of 88.9% of DDIs were found in polymedicated patients. Therefore, the likelihood of DDIs was 4.52 higher in polymedicated patients compared to those taking fewer drugs. However, this difference was not significant (p=.05). In total, 66.7% of DDIs were found in patients admitted for chemotherapy treatment (mean=0.5 DDIs/patient [95% CI, 0.05–0.95]). However, in patients admitted for other reasons, there was a mean of 0.125 DDIs/patient (95%CI, −0.01–0.26). Consequently, chemotherapy patients are 4 times more likely to experience DDIs. However, the comparison of means was not significant (p=.07).
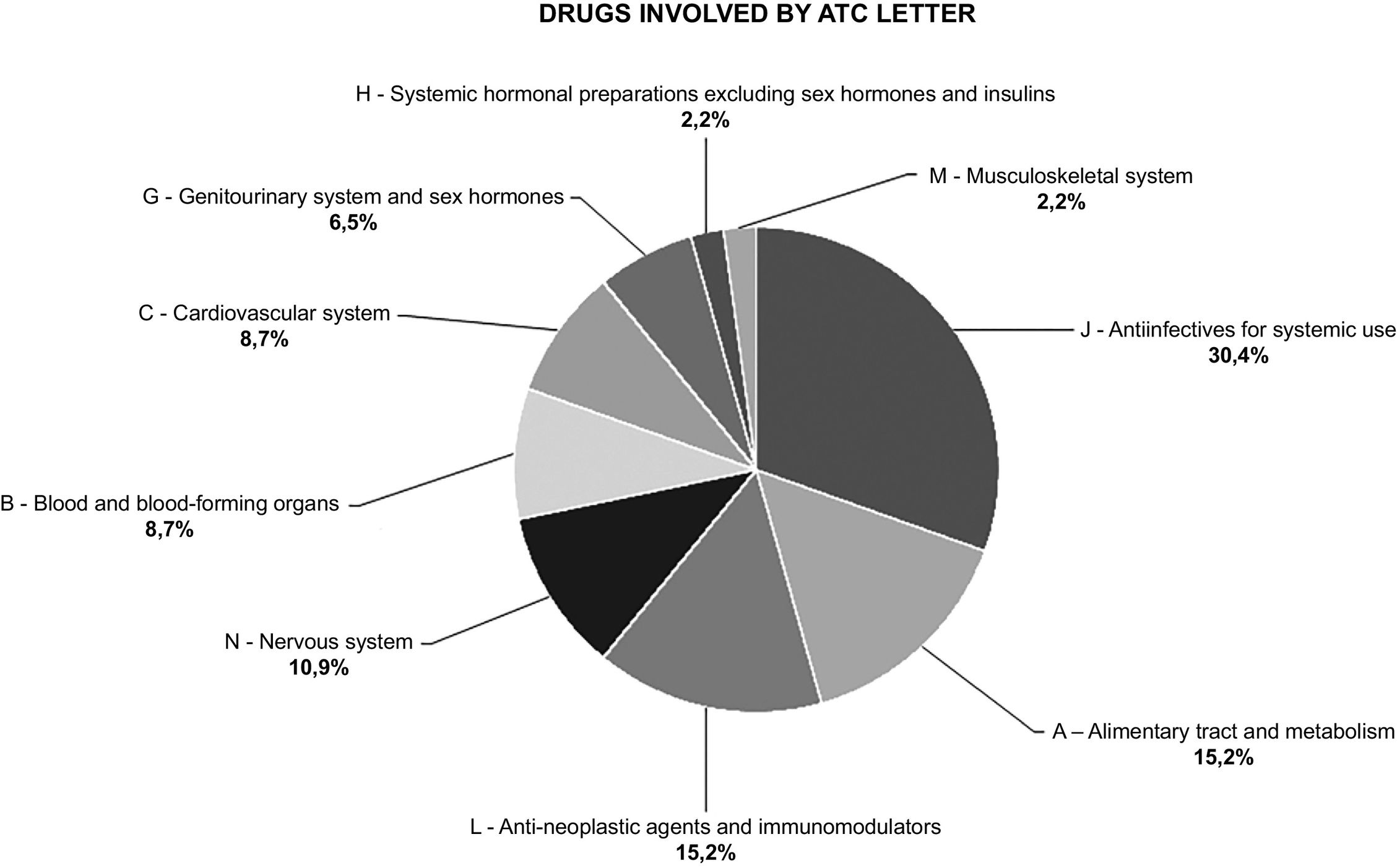
A total of 46 drugs were involved in the UDs with accepted PI. All UDs involved a single drug, except those involving a DDI, where a second drug was also involved. Fig. 3 shows drugs grouped according to their ATC letter.
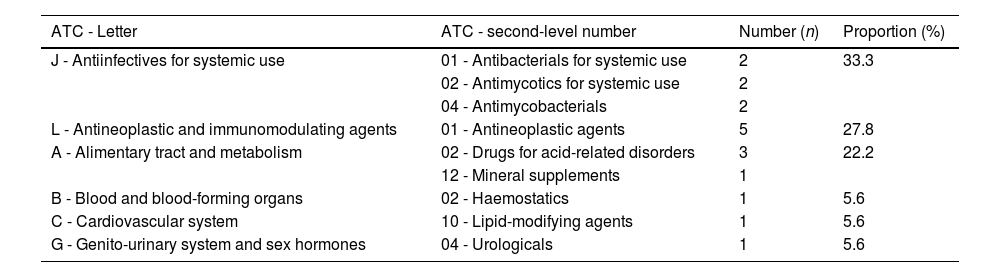
Seven of the drugs involved in the UDs belonged to ATC group L01 (antineoplastics). Of these, 71.4% had a UD involving a DDI. Table 2 shows the ATC classification of drugs involved in clinically relevant DDIs; Appendix 2 shows further details of the drugs involved and nature of the interaction.
Classification of the drugs involved in the clinically relevant DDIs detected by ATC group.
| ATC - Letter | ATC - second-level number | Number (n) | Proportion (%) |
|---|---|---|---|
| J - Antiinfectives for systemic use | 01 - Antibacterials for systemic use | 2 | 33.3 |
| 02 - Antimycotics for systemic use | 2 | ||
| 04 - Antimycobacterials | 2 | ||
| L - Antineoplastic and immunomodulating agents | 01 - Antineoplastic agents | 5 | 27.8 |
| A - Alimentary tract and metabolism | 02 - Drugs for acid-related disorders | 3 | 22.2 |
| 12 - Mineral supplements | 1 | ||
| B - Blood and blood-forming organs | 02 - Haemostatics | 1 | 5.6 |
| C - Cardiovascular system | 10 - Lipid-modifying agents | 1 | 5.6 |
| G - Genito-urinary system and sex hormones | 04 - Urologicals | 1 | 5.6 |
ATC, Anatomical/Therapeutic/Chemical Classification System.
None of the notified DDIs involved 2 drugs from the same group. Systemic antiinfectives were involved in 66.7% of the DDIs, antineoplastic drugs in 55.6%, and alimentary tract and metabolism drugs in 44.4%.
Two key areas for improvement were identified with regard to the TRP: firstly, the need to perform Med Rec at discharge, and secondly, the requirement to increase the number of TRPs.
DiscussionA number of institutions, including the ISMP, recommend that Med Rec be performed as a rigorous and standardized practice to minimize MEs during care transitions. During the study period, the TRP was performed for more than one-third of the patients admitted to the haematology department of our hospital. This figure is considerably below the optimal target of achieving a TRP for 100% of admitted patients. It should be noted that the study period included the summer holiday period, which may have resulted in a loss of patients lost to the study.
The majority of Med Recs procedures were performed within 24 hours of admission, in accordance with the recommendations set forby the ISMP.12 The primary reason for delayed Med Recs was the admission of patients on weekends and bank holidays.
The percentage of patients with UDs identified in this study is consistent with the results of other studies, although this percentage exhibits significant variability due to differences in the populations analysed, the terminology used, and the detection methods employed.3,4,14,19 Medication omission was the most frequent type of UD. This finding is consistent with the results of other studies that have identified medication omission as the primary UD, with reported percentages ranging from 42% to 71.3%.3,4,19,20 The majority of studies have identified discrepancies in dose, route, or frequency as the second most common type of UD. However, in our study, DDIs constituted the second most common UD, accounting for almost a quarter of all UDs. This discrepancy may be due to the type of population studied. The prevalence of DDIs has been reported to be significantly lower in non-haematological patients.3,4,19,20 In our study, half of patients admitted for chemotherapy treatment experienced a clinically relevant DDI. This finding is consistent with those reported by other authors, who found that between 30% and 61% of oncology patients undergoing chemotherapy experienced some clinically relevant DDI.8,13 Our study demonstrated that patients admitted for chemotherapy treatment were 4 times more likely to experience DDIs than patients admitted for other reasons. One potential explanation for this finding may be that the main drugs involved in the DDIs were systemic antiinfectives (commonly used as prophylactic treatments in haematology) and antineoplastics.8,10,13 In addition, although we did not specifically analyse the use of alternative products, this remains a particularly relevant aspect that should be considered. In our case, almost one-fifth of the patients were regularly taking herbal products, other types of supplements, or infusions, which can potentially result in DDIs with the treatments patients may be taking at home.2,13,21
Previous studies have identified polypharmacy as a significant risk factor for medication-related problems.5,7 This is a particularly pertinent issue in the context of our study given the high prevalence of polypharmacy: 63.9% of the patients were taking a minimun of five drugs. Subgroup analysis revealed that polypharmacy was associated with a 2.92-fold increase in the likelihood of UDs. Polypharmacy was also identified as a risk factor for the occurrence of clinically relevant DDIs, with a 4.52-fold increase in the likelihood of DDIs observed in patients were polymedicated. These findings are consistent with those previous studies which have identified polypharmacy and oncological treatment as factors that increase the potential risk of DDIs.8,13
The two most common PIs were medication reintroduction and the prescription discontinuation, which is consistent with the finding that the two most common types of UD were medication omission and DDIs. The level of acceptance of PIs differs considerably between studies. Some studies have reported acceptance rates of approximately 100%, a figure that is similar to that observed tin our own research, whereas others have reported acceptance rates of about two-thirds. Such variation may be attributed to discrepancies in the methodological approaches employed in these studies.5,19
Most TRPs were conducted upon admission, with lesser degree of occurrence, during in-hospital transfers. The ISMP recommends the implementation of Med Rec procedures during all stages of patient care, with particular emphasis on the discharge phase, as this crucial juncture for patients who are receiving multiple medications. This is due to the potentially greater clinical impact of MEs when patients are no longer in hospital.12 In our case, no TRP was performed at discharge during the study period, which is one of the main limitation of this study and a crucial area for improvement to be taken into account when implementing the Med Rec protocol. Two further areas meriting attention are the number of TRPs conducted and coordination with the rest of the care team.
It has been reported that pharmacists collect more comprehensive information about patients' medications than doctors.4 Haematological patients have several characteristics that increased the risk of medication-related harm. These include: (1) the need for care in a variety of settings; (2) the high prevalence of elderly patients wirh multiple medications; and (3) the requirement for treatment with high-risk drugs. The safety of this group of patients is a priority and needs to implement measure to improve it.12 Therefore, pharmacists specializing in oncohaematology should seize the opportunity to systematically provide appropriate, standardized Med Rec for all oncohaematology patients during various care transitions. Nevertheless, this complex activity should be undertaken by a multidisciplinary team applying agreed-upon protocols that optimize and clearly define the processes and responsibilities of the various departments involved in TRPs.2,3,5,14,15,20,21
The main limitations of the study are the short study period, the limited number of patients, and issues associated with its cross-sectional design. A consensus on the key concepts of the TRP would facilitate comparative analyses between studies and improve the interpretation of results. Further high-quality studies are required to elucidate the real potential of Med Rec to improve health outcomes, reduce MEs, decreasing emergency department visits, and alleviate the financial burden of care.
In conclusion, the most common UDs in TRPs were medication omissions and DDIs, while the most common PIs were medication reintroduction and prescription discontinuation. Polypharmacy was found to be associated with an increased likelihood of UDs. The administration of chemotherapy and polypharmacy were identified as the primary factors contribuiting to the increased prevalence of DDIs. A crucial area for enhacement was identified, namely the necessity to implement a procedure to guarantee that Med Rec is performed at the time of discharge.
Contribution to the scientific literatureThis pilot study is notable for its focus on the population studied, as there are virtually no publications on Med Rec in haematological patients. The results of this study differ from studies on the TRP in non-haematological patients, due to the unique characteristics of this population (frail patients with comorbidities and polypharmacy) coupled with the treatments used in this context.
These results suggest that, in clinical practice, clinical pharmacists specializing in oncohaematology should lead the Med Rec process for haematological patients admitted to the hospital. This approach has the potential to reduce the incidence of medication errors, particularly those related to DDIs, polymedicated patients, and those undergoing chemotherapy.
Ethical responsibilitiesThe present study (CEIM registration number 201/2022) was approved by the Medical Research Ethics Committee of the Institut d'Investigació Sanitària Pere Virgili (26 January 2023; minute number 001/2023).
FundingNone declared.
CRediT authorship contribution statementAlejandro Sanjuán Belda: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. María Vuelta Arce: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jorge del Estal Jiménez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Formal analysis, Data curation. Laura Canadell Vilarrasa: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Formal analysis, Data curation.