To analyze the role played by the clinical pharmacist and its impact in antibiotic stewardship facing suspected allergy to beta-lactam antibiotics.
MethodWe performed 2 different independent bibliographic searches. A total of 35 articles were found, and the final number included in the study was 12. We analyzed the articles and collected variables of efficacy, safety, and applicability of evaluation tools applied to patients with suspected allergy to beta-lactams. Also, the variation in the consumption and prescription profile of alternative antibiotics was analyzed.
ResultsThe selected studies analyzed questionnaires, allergy delabeling, intradermal tests, and oral challenge tests performed by pharmacists.
Significant differences in the efficacy endpoint were found in 4 studies in favor of pharmaceutical intervention. In the study of Kwiatkowski et al., cefazolin use increased in surgical patients after pharmacist intervention (65% vs 28%; P < .01). In a quasi-experimental study, the mean defined daily dose of aztreonam and the mean days of therapy per 1000 patients/day decreased (21.23 vs 9.05, P <.01) and (8.79–4.24, P = .016), pre- and post-intervention, respectively, increasing antibiotic de-escalations (P = < .01). In another quasi-experimental study, the prescription of restricted use antibiotics decreased (42.5% vs 17.9%, P < .01)and the use of pre-surgical prophylactic antibiotics alternative to cefazolin (81.9% vs 55.9%, P < .01)in another study.
Other study showed that the mean time per interview was 5.2 min per patient. No adverse events were reported in any study.
ConclusionThe pharmacist intervention in the evaluation of the patient with suspected allergy to beta-lactams is effective, safe, and feasible to implement on daily clinical practice. The standardization of protocols to clarify the history of allergies and development of evaluation tools represent simple screenings to perform delabeling or refer to the Immunoallergology service, improving penicilins use and reducing the need for second-line antibiotics.
More studies are needed to standardize the desensitization tests made by pharmacists.
However, despite these results, the involvement and leadership of the pharmacist in this area is limited and constitutes a future challenge for the profession.
Analizar el papel desempeñado por el farmacéutico clínico y su impacto en el ámbito de los programas de optimización de antimicrobianos ante la sospecha de alergia a antibióticos beta-lactámicos.
MétodoSe realizaron 2 búsquedas bibliográficas independientes. Se encontraron un total de 35 artículos incluyéndose 12. Se analizaron los artículos incluidos y se recogieron variables de eficacia, seguridad y aplicabilidad de herramientas de evaluación a pacientes con sospecha de alergia a beta-lactámicos. Además, se analizó la variación en el consumo y en el perfil de prescripción de antibióticos alternativos.
ResultadosLos estudios seleccionados analizaron cuestionarios, desetiquetado, test intradérmicos y pruebas de provocación oral realizados por farmacéuticos.
Se hallaron diferencias significativas en la variable principal de eficacia en 4 estudios incluidos a favor de la intervención farmacéutica. En un estudio cuasi experimental, la utilización de cefazolina aumentó tras intervención farmacéutica (65% vs 28%; p < 0.01). En otro estudio cuasi experimental, la dosis diaria definida media de aztreonam y la media de días de terapéutica por 1000 pacientes/día disminuyeron (21.23 vs 9.05, p < 0.01) y (8.79–4.24, p = 0.016), pre y post intervención, respectivamente, aumentando las desescaladas antibióticas (p = < 0.01). En otro estudio, disminuyó la prescripción de antibióticos de uso restringido (42.5% vs 17.9%, p < 0.01) y en otro, la utilización de antibióticos profilácticos pre-quirúrgicos alternativos a cefazolina (81.9% vs 55.9%, p < 0.01).
En otro estudio, el tiempo medio por entrevista fue de 5.2 minutos por paciente. No se reportaron eventos adversos en ningún estudio.
ConclusionesLa intervención del farmacéutico en la evaluación del paciente con sospecha de alergia a beta-lactámicos resulta eficaz, segura y aplicable a la práctica clínica. La estandarización de protocolos para esclarecer la historia de alergias y desarrollo de herramientas de evaluación constituyen un sencillo cribado para realizar desetiquetado o referenciar al servicio de inmunoalergologia, mejorando el uso de penicilinas y reduciendo la necesidad de antibióticos de segunda línea. Son necesarios más estudios para uniformizar la realización de tests de desensibilización por farmacéuticos.
Sin embargo, a pesar de estos resultados, la implicación y liderazgo del farmacéutico en esta área es escasa y constituye un desafío futuro para la profesión.
β-lactam antibiotics are the most widely used drugs in the treatment of infectious diseases due to their efficacy, spectrum of activity, and safety. Their prescription can be limited by bacterial resistance and the appearance of adverse reactions, among which hypersensitivity is of special relevance. Penicillin allergy is the most commonly reported type of drug allergy.1
About 10% of patients report a history of allergy; however, it is estimated that less than 1% of the population is actually allergic. According to the American Academy of Allergy, Asthma, and Immunology, 80% of patients with IgE antibody-mediated penicillin allergy lose sensitivity after 10 years.2
Of note, the rate of cross-reactivity between penicillins and cephalosporins is less than 1%.3
Nevertheless, β-lactam therapy is often completely avoided in patients with no real allergies or with mild reactions.
Of greater relevance are IgE-mediated allergic reactions (type I allergic reactions), which usually occur within the first hour of drug administration. Symptoms include urticaria, angioedema, shortness of breath, wheezing, and anaphylaxis. Other severe reactions, which are not IgE-mediated (late allergic reactions, type II, III, and IV), include Stevens-Johnson syndrome, toxic epidermal necrolysis, interstitial nephritis, hemolytic anemia, acute serum sickness, or drug sensitivity syndrome with eosinophilia and systemic symptoms (DRESS).
The unwarranted avoidance of β-lactam antibiotics is of concern in several respects: it may lead to the use of potentially less effective antibiotics (e.g. vancomycin is less effective than cloxacillin against methicillin-sensitive Staphylococcus aureus), and it may promote the use of agents with a broader spectrum of activity and a higher likelihood of resistance development, potentially leading to an increased risk of toxicity (e.g. the selection of Clostridioides difficile, [CD] and methicillin-resistant S. aureus). Furthermore, poorly characterized allergies are associated with delays in the first administration of antibiotic therapy, higher rates of rehospitalization and prolonged hospitalizations, an increased risk of surgical site infection, increased adverse reactions, mortality, and higher associated costs.4
Pharmacists as agents in antimicrobial stewardship programsAntibiotic resistance is a worldwide public health problem. It has been estimated that in the United States, 2.8 million infections are caused by resistant bacteria every year resulting in over 35 000 deaths.5 It has also been estimated that in the European Union, 670 000 infections are caused by resistant bacteria each year resulting in 33 000 deaths.6
The inappropriate use of antibiotics has been found to be the most relevant modifiable risk factor for resistance development. Therefore, the promotion of the appropriate use of antibiotics in all clinical settings is essential, and the pharmacists' role is integral to achieving this goal. Antimicrobial Stewardship Programs (AMSP) comprise a set of strategies that promote the appropriate use of antibiotics. These strategies attempt to optimize the effectiveness of antibiotics, minimize adverse events and toxicities associated with their use, limit the development of resistant bacteria, and reduce unnecessary costs associated with the excessive and inappropriate use of antibiotics.7–10
Pharmaceutical organizations such as the American Society of Health-System Pharmacists (ASHP) and the Society of Infectious Diseases Pharmacists (SIDP) have published guidelines on the role of pharmacists in AMSPs in hospital and outpatient settings, respectively.11–13
Currently, establishing protocols for managing suspected β-lactam allergies is a high priority for hospital infection control groups; however, despite their multidisciplinary profile, the role of pharmacists remains underdeveloped in these groups.
The main objective of this review was to analyze the role played by clinical pharmacists and its impact on AMSPs in the setting of suspected allergy to β-lactam antibiotics.
Material and methodsLiterature searchA bibliographic search for MeSH terms was performed in the main biomedical databases: MEDLINE (PubMed) and EMBASE. The strategies and terms used in the searches were as follows: (“β-Lactams” [Mesh] AND “Hypersensitivity” [Mesh]) AND “Pharmacists” [Mesh]) AND/OR “Antimicrobial Stewardship” [Mesh]. The years 2018–2022 (last 5 years) was established as the search period. A literature search was also conducted by the Centro de Informação do Medicamento da Ordem Dos Farmacêuticos de Portugal (CIM-OF).
Selection of studiesThe studies were selected according to the following criteria:
Inclusion criteria: studies were included that: (a) described the role played by clinical pharmacists in AMSPs in the setting of suspected allergy to β-lactam antibiotics; (b) evaluated the clinical parameters of patients in terms of efficacy and/or safety; (c) analyzed the feasibility of the procedures implemented by pharmacists; and (d) described information about changes in the pattern of antibiotic use, cost, hospital stay, and/or patient perception.
Exclusion criteria: studies were excluded that: (a) did not include pharmacist participation; (b) only analyzed differences in the pattern of antibiotic prescription; (c) only analyzed costs; (d) were not written in Spanish, Portuguese, or English; and (e) studies were excluded for which the complete text could not be located. Regarding the last 2 criteria, no studies have analyzed or described the role played by clinical pharmacists in AMSPs in the setting of suspected allergy to β-lactam antibiotics.
Data analysisFollowing the selection procedure, a team of 2 researchers independently analyzed the studies and collected data on the following variables: country of publication, design, pharmaceutical intervention, patient sample, and results of the intervention (efficacy, safety, and/or other variables such as applicability or analysis of consumption).
The data were then pooled by consensus and, in its absence, a third researcher participated in the procedure.
The studies were selected and analyzed using the Prisma 202014 guideline as a reference.
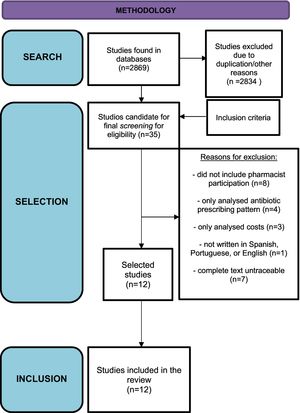
ResultsIn total, 35 studies were identified that met the search criteria. After applying the inclusion and exclusion criteria, 12 studies were finally selected. Fig. 1 shows the study selection process.
A number of studies were excluded on the following grounds: 8, because they did not include pharmacist participation; 4, because they only analyzed differences in the pattern of antibiotic prescription; 3, because they only analyzed costs; 1, because it was not written in Spanish, Portuguese, or English; and 7, because the complete texts could not be located.
Of the included studies, 11 were quasi-experimental studies and 1 was an observational study.
Table 1 shows the results of the studies.
Results of the studies.
| Author, year, country | Design | Pharmaceutical intervention | n (sample) | Direct de-labeling | Oral challenge/intradermal test | Referral to IA service | Consumption of ABs | Safety |
|---|---|---|---|---|---|---|---|---|
| Tanya du Plessis et al.15J Antimicrob Chemother 2018 (New Zealand) | Quasi-experimental | Structured questionnaire, de-labeling, and oral challenge | 250 adults, admitted | 64%, of which 50% had already tolerated a penicillin-containing antibiotic | 12.4% | 20% (47% with confirmed allergy) | N/A | No adverse effects reported in 98% of patients after 1 year of follow up |
| Mitchell et al.16Fed Pract 2021 USA | Observational | Structured questionnaire, de-labeling, and oral challenge | 278 adults, admitted | 22% | 8.6% | N/A | N/A | N/A |
| Harmon S et al.17Hospital Pharmacy 2020 (USA) | Quasi-experimental | Intradermal test | 31 adults, admitted | N/A | 96% | N/A | Average daily savings of US $74.75 per patient | 2 patients with adverse effects after intradermal test: skin rash and local reaction |
| Turner NA et al.18JAMA, 2021 (USA) | Quasi-experimental: case control | Structured questionnaire, de-labeling, penicillin skin testing and interpretation, referral to IA | 273 adults, admitted | 17.2% | 68% | 0.4% | Reduced use of alternative and high CD infection-risk antibiotics after applying the questionnaire (RR 0.87; 95%CI, 0.79–0.97) and (0.91; 95%CI, 0.85–0.98), respectively | N/A |
| Mann KL et al.19J Antimicrob Chemother 2019 USA | Quasi-experimental | Structured questionnaire, direct de-labeling | 175 adults | 1.1% (at patient's own request)+13% | N/A | N/A | N/A | No adverse effects reported |
| Clark KE et al.20J Pharm Pract 2019 (USA) | Quasi-experimental | Structured questionnaire | 95 adults preintervention vs 65 postintervention | N/A | N/A | N/A | Decrease in DDD aztreonam/1000 patients/d: 21.23 vs 9.05; P = 0.003) | No adverse effects reported |
| Devchand M et al.21J Antimicrob Chemother 2019 (Australia) | Quasi-experimental | Structured questionnaire, de-labeling, oral challenge, and skin prick testing | 106 adults, admitted | 13% | 18.8% (oral) 3.8% (skin) | 25.4% | N/A | One patient reported late cutaneous rash in response to oral amoxicillin challenge; new record added to patient's allergy history |
| Vaisman et al.22J Antimicrob Chemother 2017 (Canada) | Quasi-experimental | Structured questionnaire | 485 adults, prior to elective surgery | N/A | N/A | N/A | Decrease in AB alternatives, 81.9% vs 55.9% (P = .001) pre-postintervention | No adverse effects reported after cefazolin administration |
| Ham Y et al.23Allergy Asthma Proc 2021 (USA) | Quasi-experimental | Structured questionnaire and oral challenge | 50 adults, admitted | 40% | 56% | N/A | N/A | 2 patients with mild adverse effects after oral challenge |
| Louden NJ et al.24J Pediatr Pharmacol Ther, 2021 USA | Quasi-experimental | Structured questionnaire, direct de-labeling, and referral to IA | 11 pediatric patients | 18% | N/A | 82% | N/A | N/A |
| Kwiatkowski S et al.25Am J Health Syst Pharm 2021 (USA) | Quasi-experimental: case control | Telephone questionnaire prior to elective surgery | 87 adults | N/A | N/A | N/A | Use of augmented cefazolin (P = .001) 65% vs 28% | N/A |
| Song YC et al.262021 USA | Quasi-experimental | Structured questionnaire | 66 adults, admitted | 18% | N/A | N/A | N/A | N/A |
IA, immuno-allergology service; AB, antibiotic; N/A, non-applicable/no mention of result; CD, Clostridioides difficile; 95%CI, 95% confidence interval; DDD, defined daily dose.
In 11 studies, the pharmacist interviewed the patient with suspected β-lactam allergy, collected the complete history of the allergic reaction and evaluated it using standardized questionnaires15,16,27; in 1 study, skin tests were performed without prior interview.17
The interventions performed after the interview were as follows: direct de-labeling (6 studies); oral challenge tests (4 studies); skin prick/intradermal tests in inpatients or outpatients (3 studies); and referral to the immuno-allergology service (2 studies).
In 10 studies, interventions were performed in hospitalized patients with records of β-lactam allergy. Only 2 interventions were performed on patients who had not yet been admitted prior to elective surgical procedures, and only 1 study included pediatric patients.
Identification of patients who were candidates for interviewCandidates were identified through entries of suspected allergic reaction to β-lactams in their electronic medical record.
Some studies refer to the creation by pharmacists of automated tools to facilitate daily searches for these patients.16,17,19,26
One study included patients pending elective surgery with reported allergies, who were expected to be given β-lactam antibiotic prophylaxis.25 Patients meeting criteria were informed of the completion of the questionnaire 1 week before pre-operative anesthesia consultation.
Questionnaires, risk stratification, and previous trainingThe structured questionnaires referred to in the studies collected information on the antibiotics involved, characteristics of the reactions, time since they occurred, and any subsequent administrations of β-lactam antibiotics to confirm that they were well-tolerated.16,19–21,24,26
Subsequent risk was stratified at 3 or 4 levels: no risk, low-, moderate-, and high risk. Each category was associated with a recommended action: de-labeling, oral challenge, skin test followed by challenge, or avoidance of β-lactams.
The results of the questionnaires and the recommendations were recorded in the electronic medical records.16,19–21,24,26
In 1 study, an electronic assessment tool was created that removed information from the clinical process and automatically categorized the level of risk.24
Several studies refer to previous pharmacist training of varying duration,20,24 which was given by infectionists22 or immuno-allergologists,23 who were always available to resolve questions. Training comprised 2 components: theoretical and practical, with case-by-case discussion.
Skin testingA previous study17 described the skin tests, which comprised 3 steps: a skin prick test; an intradermal test with diluted penicillin; and a challenge dose of oral amoxicillin 250 mg or intravenous ampicillin if patients were intolerant to oral administration. These tests were performed by resident pharmacists and previously trained specialists, and followed a protocol approved by the Pharmacy and Therapeutics Commission.
Previous studies identified potential drugs that could mask histamine release.16
De-labelingWhen de-labeling was indicated, the AMSP pharmacist recorded the result of the assessment and updated the allergy profile in the electronic medical record.16,18,21 In 1 study, this information was added to the original medical record.16 In some cases, the patients were provided with a card to alert family physicians of this update.18
EfficacyEfficacy variablesThe efficacy of the intervention were assessed using the following main outcome measures: percentage of de-labeling (in 8 studies); antibiotic consumption (β-lactam or alternative) (in 4 studies); CD rate (in 1 study); and overall mortality (in 1 study).
The following efficacy variables were also included: the number of patients whose antibiotic regimen was de-escalated; the cost of antibiotic treatment; and the probability of prescribing β-lactams, first-generation cephalosporins, or high CD infection-risk antibiotics.
De-labelingThe percentage of patients who underwent direct de-labeling (following clarification of their allergy history via interview) ranged from 13% to 64% (median: 18%). The study by du Plessis et al.15 had the highest percentage of de-labeling; 50% of patients had tolerated 1 course of a penicillin antibiotic prior to inclusion.
After skin testing or oral challenge, the de-labeling rates ranged from 8.6%16 to 96%.17
Antibiotic consumptionIn 4 of the studies, significant differences were found in the primary endpoint in support of the pharmaceutical intervention.
There was a decrease in the mean defined daily dose (DDD) of aztreonam/1000 patient-days and mean days on therapy/1000 patient-days (21.23 vs 9.05; P < .01) and (8.79–4.24; P = .016); these results are based on pre- and post-implementation data obtained from the questionnaire and associated recommendations, respectively.20 A significant decrease was found in the prescription of restricted-use antibiotics (42.5 vs 17.9%; P < .01).21 A decrease was reported in the use of pre-operative prophylactic antibiotics alternative to cefazolin (81.9 vs 55.9%; P < .01).22 An increase was found in cefazolin use from 28% to 65% (P < .01).25 No significant differences were found between pre- and post-intervention periods in the consumption of narrow-spectrum β-lactams (amoxicillin, amoxicillin–clavulanic acid) and restricted-use antibiotics, including high CD infection-risk antibiotics (e.g., ceftazidime, ceftriaxone, ciprofloxacin, and clindamycin).18 The only difference was related to the decreased consumption of antibiotics alternative to penicillins (aztreonam, ciprofloxacin, etc), with a higher probability of β-lactams being prescribed before discharge.
SafetySafety variablesTwo studies found no adverse events during the study period,19,20 and 1 study found no adverse events at 1 year follow-up.15 In total, 98% percent of patients who were de-labeled had no adverse events after repeated administration of penicillin-containing antibiotics. Likewise, 1 study found no adverse reactions after administration of cefazolin.22
However, some of the studies reported adverse effects: 1 patient developed late rash21; 2 patients experienced mild adverse effects after oral challenge23; and 2 patients experienced adverse effects (skin rash and local reaction) after intradermal testing.17
Other parametersThere was a mean decrease in overall antibiotic therapy costs ($74.75/d).17
The mean time spent per interview was 5.2 min/patient.26
DiscussionEstablishing protocols for managing suspected β-lactam allergies is one of the main priorities for hospital infection control groups. Several recent publications have analyzed the efficacy, safety, and cost-effectiveness of such measures.24–26
Clinical pharmacists are members of these groups and, together with the rest of the team, are fully involved in this activity; however, there are few published reports of such activity being led by these professionals.
In our review, the main pharmaceutical interventions were structured interviews (telephone or face-to-face) with patients with suspected allergy, skin tests, and oral challenge tests under surveillance, direct de-labeling of patients considered non-allergic after confirmation of the clinical history, and referral to immuno-allergology specialists for further specific tests.
After analyzing the results of studies reviewed, we found that they offer a certain degree of guidance or recommendations on the best course of action to take in patients with suspected allergies. However, they are not decisive or of sufficient scientific robustness to decide whether pharmacists are appropriate healthcare staff to provide leadership in this role.
Despite the lack of conclusive results, pharmacists have increasing presence and visibility in infection control groups, which is a situation endorsed by both international and national regulations. For example, in Portugal, the regulation Direçao geral de Saude (DGS) of September 202228 requires the implementation of such groups in both public and private hospitals with the aim of promoting activity related to AMSPs and reducing the risk of antimicrobial resistance. It is mandatory that such groups include at least 1 pharmacy specialist.
In light of the results, simple measures such as computer tools or patient questionnaires to clarify histories of allergic reactions29 can be used to de-label “low-risk” patients and refer them to immuno-allergologists, thus improving the use of penicillins and reducing the need for second-line antibiotics. This approach is likely to reduce the risk of iatrogenic and multidrug-resistant infections, and reduce healthcare costs.
However, there is no consensus on clinical decision rules for de-labeling and classifying “false” allergies as intolerances. Furthermore, many of these decision rules, such as Pen-Fast,29 are not specifically validated for use by healthcare staff other than physicians.
Although previous studies have reported pharmacists conducting oral penicillin challenge and desensitization skin tests, these are very specific cases in that the pharmacists received specialized training by immuno-allergologists. However, no details on such training are provided regarding the objectives, duration, or competencies to be acquired.15–18,21,23,30,31 Furthermore, the highly heterogeneous nature of the health and policy frameworks in which these studies were conducted makes it difficult to extrapolate such training into the daily practice of hospital pharmacists.
In the case of Spain and Portugal, this activity is conducted by immuno-allergologists.
In addition, of the 12 studies reviewed, only 7 refer to safety variables and the outcomes are not defined. However, the number of adverse effects reported was low and those reported were considered to be mild.
This review offers detailed and current information from published studies, specifically focussing on the role of well-trained clinical pharmacists. It assesses how this role impacts patients with suspected or reported β-lactam allergies, particularly in terms of directly de-labeling or referring patients to immuno-allergology specialists.
LimitationsThe main limitation of this review is that no randomized clinical trials are available. Furthermore, some of the conclusions were based on low-quality quasi-experimental studies, the review did not evaluate any potential biases within the studies, and, after the exclusion were applied, the final number of studies was quite low.
Another relevant limitation is that only 2 databases were used for the literature search. In order to obtain the most recent evidence on the study topic, the period 2018–2022 (last 5 years) was established as the search period; however, an additional search was conducted by the CIM-OF to minimize the risk of inclusion or selection bias.
ConclusionThe latest scientific evidence suggests that involving pharmacists in the evaluation of patients suspected of having β-lactam allergies is effective, safe, and applicable in clinical practice. Standardized protocols to clarify allergy histories and evaluation tools, such as structured questionnaires, would provide a straightforward screening method for de-labeling or referral to an immunology/allergy service in specific situations. Thus, patients with false allergies could be de-labeled and first-line antibiotics could be used safely. In addition, there would be a decrease in the consumption of alternative antibiotics, which carry a higher risk of the resistance development.
However, these potential benefits are based on highly heterogeneous studies employing low-quality methodology. Randomized clinical trials with sound methodology are needed to provide higher quality results based on the available evidence.
Specific training in this area would enable pharmacists to provide added value and broaden the range of competencies within our specialization, both in Portugal and Spain. This aspect represents a future challenge for the pharmaceutical profession.
FundingNone declared.
Author contributionsJesus Cotrina Luque, Maria José Rei, and Miriam Capoulas contributed to the conceptualization, design, definition of the intellectual content, preparation, review, and editing of the manuscript, and assume responsibility of the work.
Jesus Cotrina Luque and Maria José Rei contributed to the literature search and data collection and analysis.