One year after the declaration of the SARS-CoV-2 pandemic, only dexamethasone has clearly shown a reduction in mortality for COVID-19 hospitalized patients. For interleukin-6 inhibitors, results are variable and unclear. The objective was to review and analyze the effect of tocilizumab and sarilumab on survival in this setting.
MethodThe PRISMA statements were fulfilled for the systematic review. A systematic search in Medline, Embase and medRxiv was conducted to identify randomized controlled trials with tocilizumab or sarilumab in hospitalized patients with COVID-19. Mortality data from non-critical and critical patients were extracted. A random-effects (DerSimonian-Laird) meta-analysis was performed for both subgroups and the whole population using MAVIS software v. 1.1.3. Similarity and homogeneity among trials were assessed.
ResultsTwenty-five and 23 articles were identified in Medline and Embase, respectively, five were trials with tocilizumab and/or sarilumab; two more were identified at medRxiv. Seven randomized clinical trials fulfilled the inclusion criteria. Another trial was pre-published and included post-hoc. The meta-analysis, with eight randomized clinical trials and 6,340 patients, showed a benefit on mortality for interleukin-6 inhibitor (hazard ratio 0.85; confidence interval 95% 0.74-0.99), low heterogeneity (I2 = 7%), but a low similarity among studies. The results showed no differences among critical and non-critical patients. A sensitivity analysis excluding non-similar or heterogeneous studies showed different results, without benefit and with low precision of the result in non-critical patients.
ConclusionsA benefit in mortality for interleukine-6 inhibitors was found, but with important differences among the scenarios analyzed in the clinical trials. Positive results are mainly caused by two randomized clinical trials which are similar in concomitant use of steroids and very-high mortality in critical patents. Sarilumab was poorly represented in the meta-analysis. Nevertheless, an association between the benefit and the critical/non-critical condition was not found. More randomized clinical trials, mainly focused in patients at high mortality risk, are needed to confirm the benefit of interleukine-6 inhibitors for COVID-19. Sarilumab was underrepresented in the meta-analysis.
Un año después de la declaración de la pandemia por SARS-CoV-2, solo dexametasona había mostrado claramente una reducción de la mortalidad en pacientes hospitalizados por COVID-19. Los resultados de los inhibidores de interleucina 6 son diversos y poco claros. El objetivo de este trabajo es revisar y analizar el efecto de tocilizumab y sarilumab sobre la supervivencia de los pacientes en este escenario.
MétodoLa revisión sistemática siguió las recomendaciones de PRISMA. Se realizó una búsqueda sistemática en Medline, Embase y medRxiv para identificar ensayos controlados aleatorizados con tocilizumab o sarilumab en pacientes hospitalizados con COVID-19. Se recopilaron los datos de mortalidad de pacientes críticos y no críticos y se llevó a cabo un metaanálisis de efectos aleatorios (Der Simonian-Laird) para ambos subgrupos y para toda la población, usando el software MAVIS v. 1.1.3. La similitud y homogeneidad entre los ensayos fue evaluada.
ResultadosSe identificaron 25 y 23 artículos en Medline y Embase, respectivamente; cinco eran ensayos con tocilizumab y/o sarilumab; se identificaron dos más en medRxiv. En total, siete ensayos clínicos aleatorizados cumplieron los criterios de inclusión. Posteriormente, se prepublicó otro ensayo que cumplía los criterios de inclusión y se incorporó al análisis. El metaanálisis, con ocho ensayos clínicos aleatorizados y 6.340 pacientes, mostró un beneficio sobre la mortalidad para los inhibidores de interleucina-6 (hazard ratio 0,85; intervalo de confianza al 95% 0,74-0,99), con baja heterogeneidad (I2 = 7%), pero reducida similitud entre los estudios. Los resultados no mostraron diferencias entre pacientes críticos y no críticos. Un análisis de sensibilidad excluyendo estudios heterogéneos o no similares mostró resultados diferentes, sin beneficio y con baja precisión del resultado en pacientes no críticos.
ConclusionesSe encontró un beneficio en la mortalidad de los inhibidores de la interleucina 6, pero con importantes diferencias entre los escenarios analizados en los ensayos clínicos. Los resultados positivos se deben principalmente a dos ensayos que son similares en el uso concomitante de esteroides y una mortalidad muy alta en pacientes críticos. Sarilumab estuvo escasamente representado en el metaanálisis. Sin embargo, el metaanálisis por subescenarios no encontró una relación entre el beneficio y la condición de pacientes críticos/no críticos. Se necesitan más ensayos clínicos aleatorizados, principalmente enfocados en pacientes con alto riesgo de mortalidad, para confirmar el beneficio de los inhibidores de interleucina-6 en COVID-19.
The emergence and spread of severe acute respiratory syndrome-2 coronavirus (SARS-CoV-2) has caused a global health crisis. According to World Health Organization, 119,791,453 confirmed cases of coronavirus disease (COVID-19), including 2,652,966 deaths, had been reported as of 16th March 20211. In hospitalized patients with COVID-19, the reduction of mortality is an absolute priority2. Studies with remdesivir3 or tocilizumab4 have shown reductions in time of recovery; nevertheless, if the only benefit is that patients who are going to recover reduce their time to improvement, while other patients die at the same rate without any benefit, the impact of interventions on disease management would be very poor.
One year after the declaration of the SARS-CoV-2 pandemic, only dexamethasone had shown a clear reduction in mortality for COVID-19 hospitalized patients5 and is widely used. Recently, the REMAP-CAP and RECOVERY studies6,7, both randomized clinical trials (RCT) with the interleukine-6 inhibitors tocilizumab and sarilumab, showed a reduction in mortality, with the particularity that included only critically ill COVID-19 patients or those in a progressive illness.
The objective of the present study was to review the effect of tocilizumab and sarilumab on survival in critical and non-critical hospitalized patients included in the clinical trials available to date.
MethodsA systematic review was performed following PRISMA statement. A systematic search of bibliography was conducted to identify randomized clinical trials (RCTs) with tocilizumab or sarilumab in hospitalized patients with COVID-19. Medline (Pubmed), medRxiv and Embase were used for the search. The search [(tocilizumab OR sarilumab) AND COVID-19] with filters of “clinical queries/clinical studies/therapy/narrow” was launched in Pubmed. Another search [tocilizumab AND COVID-19 AND randomized] from title, abstract and author's keywords, and the same for sarilumab were launched in Embase. A complementary search for other not yet published RCTs with pre-published data was done in medRxiv. Reference screening and a complementary non-systematic web search were performed. Randomized controlled studies with tocilizumab or sarilumab in hospitalized COVID-19 patients were included. Their quality was assessed using a validated scale8; blinding was not required. Studies without survival data, without patients admitted to the hospital or without a control group using placebo or standard therapy were excluded.
Mortality data from every study were extracted by two independent investigators, and discrepancies were reviewed. In studies including several lengths of follow-up for mortality, the longest ones were selected. Intended-to treat principle was applied in order to homogenize the data. Data of mortality from critical or non-critical patients at baseline were also pulled separately, as they are two groups with very different mortality risks and at different stages of the disease, and the effect of the treatments could be different, too. A critical patient was defined as in REMAP-CAP trial, as a patient who is admitted at ICU at randomization. Results from studies with only mixed data from critical and non-critical patients without any subgroup analysis were not considered for the separate analysis. As many trials did not record the ICU admission, and mixed ICU and non-ICU patients with high-flow oxygen or non-invasive ventilator support, an amendment was made to the protocol, according with independent counselling from intensivists: in trials where ICU/non-ICU data are lacking but a subgroup analysis for respiratory support was made, patients with high-flow oxygen or mechanical ventilation (invasive or not) were pulled together with critical-ICU patients from the other studies. Patients with non-high-flow oxygen or without respiratory support were considered as non-critical.
The publication bias was assessed by funnel plotting. The similarity among the selected studies was assessed attending to the characteristics of included patients and the design of the study. A random-effects meta-analysis (DerSimonian-Laird method) of the results, using MAVIS software v. 1.1.3, was performed for the whole population and both subgroups, critical and non-critical, using the odds ratio and its 95% confidence interval [OR (95%CI)]. The random effect method was selected because of possible dissimilarity among the studies, including samples at different stages of the disease and with very different risk of mortality. If OR (95%CI) was not shown in the article, it was calculated from raw data of mortality. Heterogeneity inside both subgroups was assessed using the I2 statistic. If heterogeneity was high, the meta-analysis was considered as non-informative and other differences between studies were explored.
When a separate analysis in patients using corticosteroids or not was done, it was considered in a specific analysis. Sensitivity analysis was done excluding a singular study when it was identified as a cause of heterogeneity and its characteristics were different from the rest of the trials. As patients and designs of the RECOVERY trial were different of those included in other trials because it represents the results of a second randomization up to 21 days later, a sensitivity analysis was performed excluding this study.
The interaction probability [p(i)] of the mortality results between both subgroups was assessed using a specific calculator9 with a bivariate methodology10,11. The estimated absolute risk reductions and number needed to treat were provided to allow an assessment of the clinical relevance of the possible effect, since the odds ratio must be applied to scenarios with different mortality in order to calculate the absolute benefit. Significance level for interaction was set at p = 0.05, with 0.1 < p ≤0.05 considered as a dubious interaction level in subgroup analysis12.
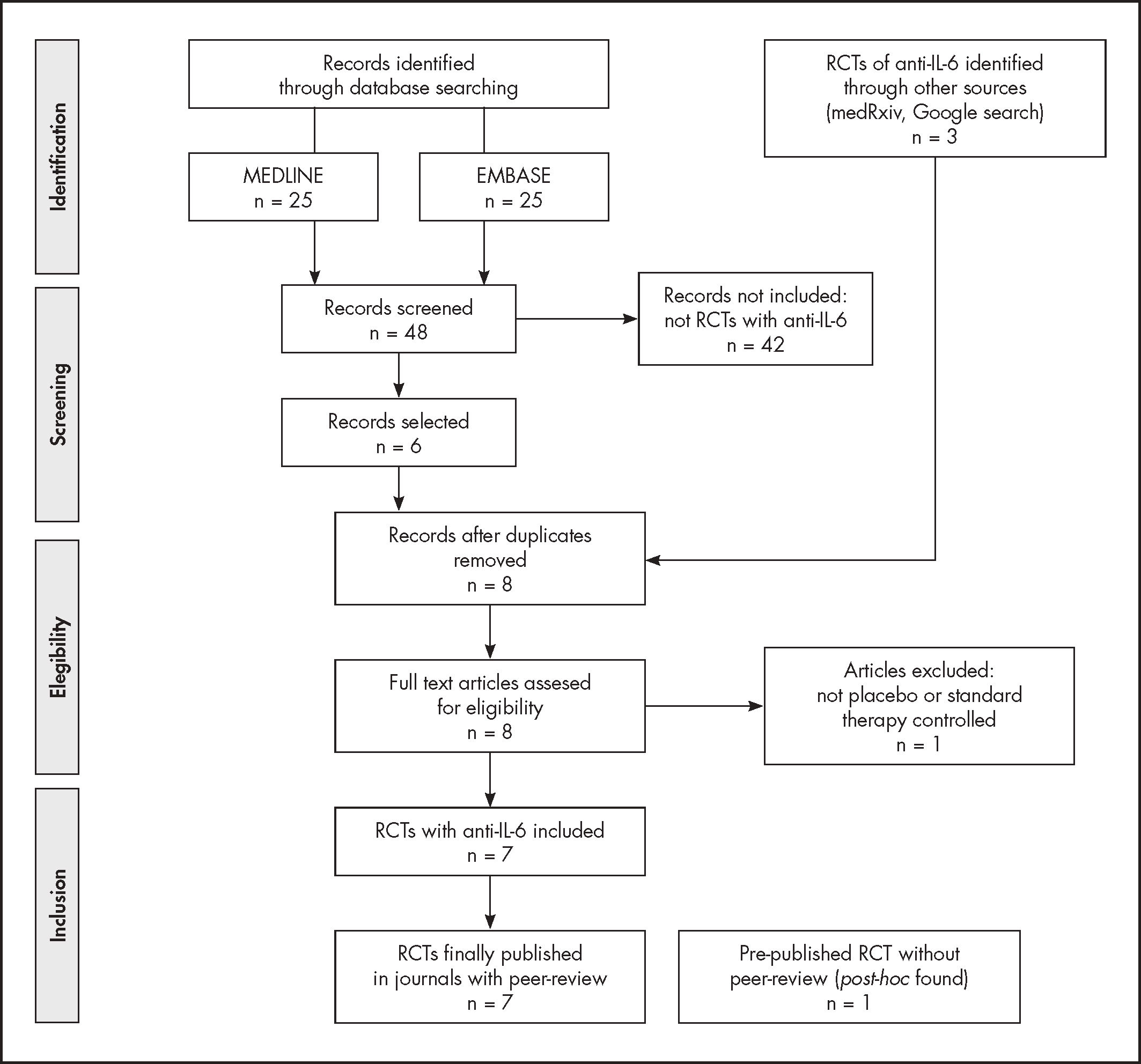
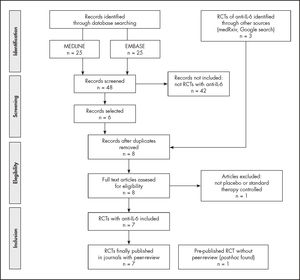
ResultsThe bibliographical search was launched in Medline (Pubmed) at Jan 27th, 2021; the details are shown in figure 1. Four RCT with tocilizumab vs. placebo or standard therapy that provided mortality data in hospitalized patients with COVID-19 were found13-16. Two more articles, not peer-reviewed, were found at medRxiv and fulfilled the criteria for inclusion. The results of these two clinical trials were finally published on February 25, 20214,6. There were no changes in the results previously reported in the preprints included in our initial review. A second search was launched in EMBASE at January 28th, 2021. Five previously identified RCTs of tocilizumab were found. Another published RCT from a non-systematic search was identified and followed the inclusion criteria17. A recent systematic review about tocilizumab in COVID-1918 with data of search on January 7th, 2021, found only two RCTs with data of mortality for tocilizumab; both were already identified by our search in Medline.
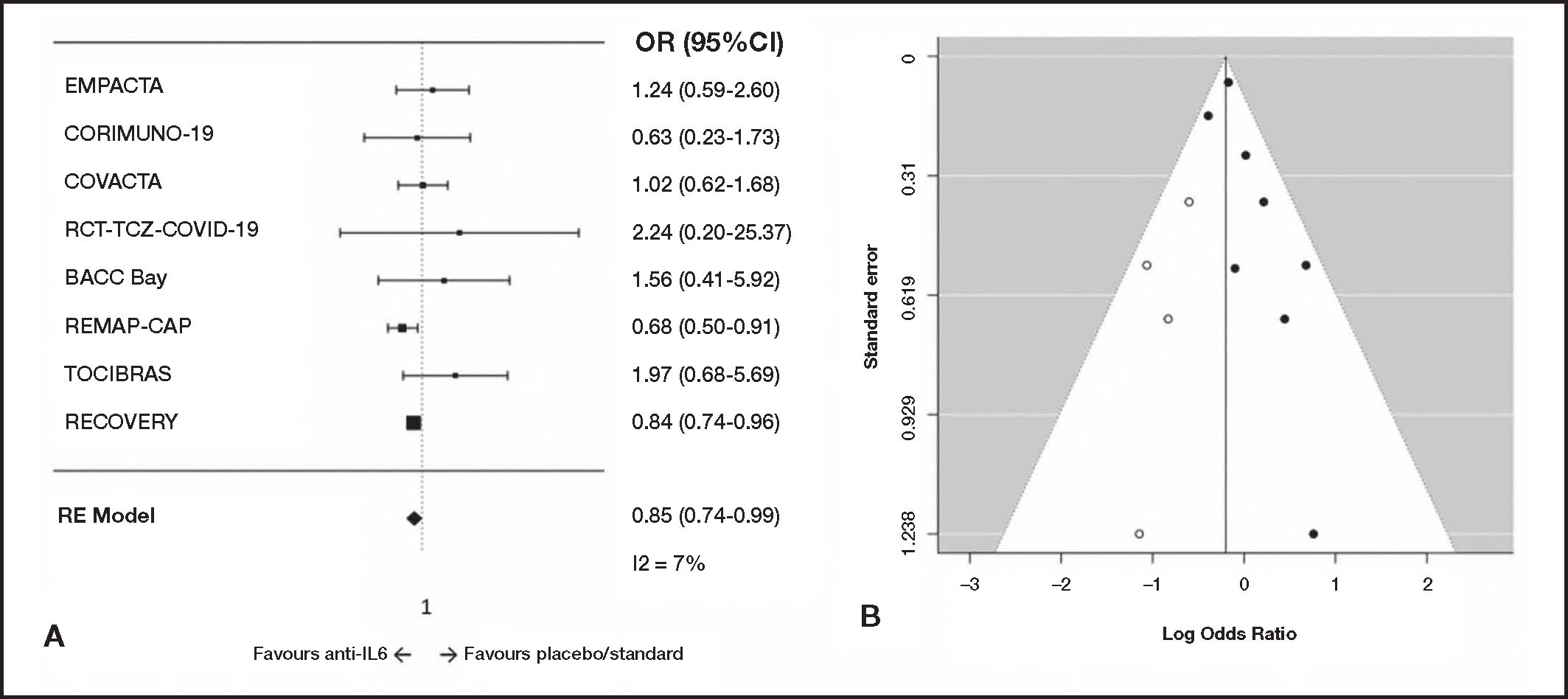
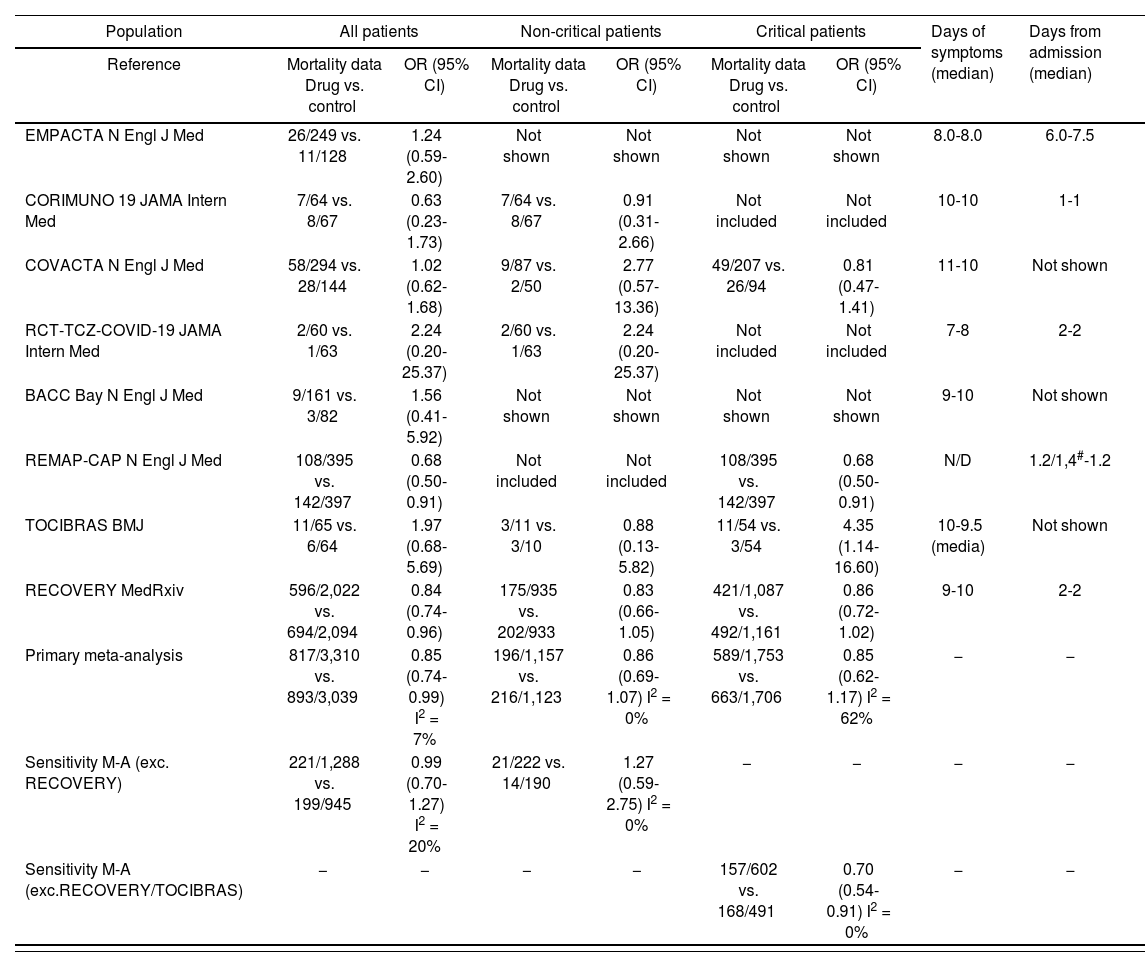
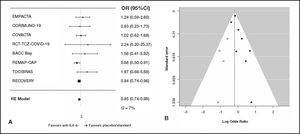
A total of seven RCTs were included. After the systematic search was made, a new pre-print of a RCT that fulfilled the inclusion criteria was published (RECOVERY)[7], and it was included in the analysis. The characteristics of the eight included studies are shown in table 1. The meta-analysis of mortality data in the whole population, with 8 RCTs and 6,349 patients, showed a low heterogeneity (I2 = 7%). The resultant OR for mortality was 0.85 (95%IC 0.74-0.99) in the whole population and 0.99 (95%CI 0.70-1.27) in the sensitivity analysis excluding the RECOVERY trial (Table 2 and Figure 2). The trim and fill method showed an inverse publication bias, with lacking little studies with more reduced odds ratios.
Randomized clinical trials of interleukin-6 inhibitors in hospitalized patients with COVID-19 (systematic review); included critical (with high-flow oxygen or invasive/no invasive mechanical ventilation) and non-critical patients
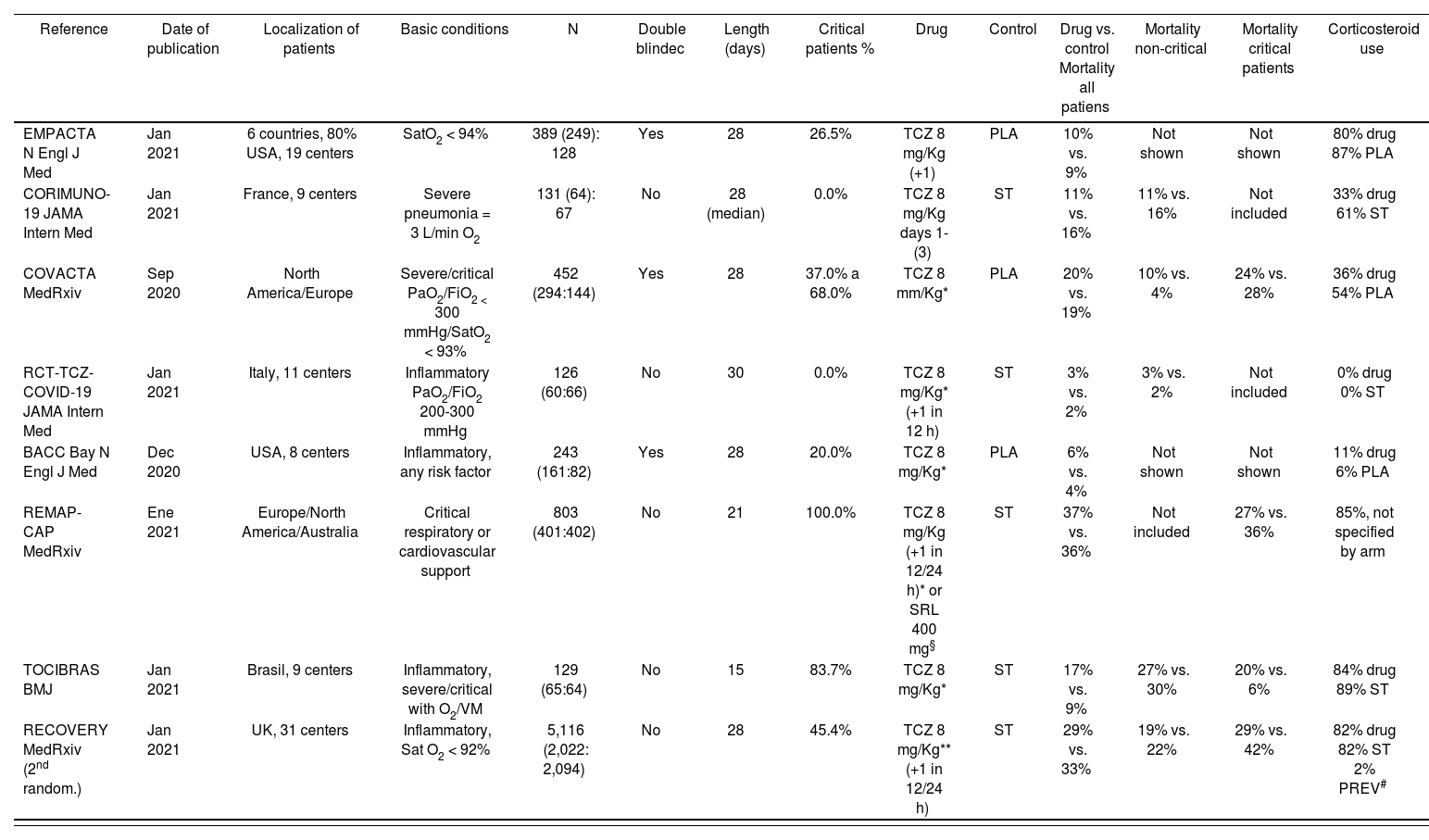
| Reference | Date of publication | Localization of patients | Basic conditions | N | Double blindec | Length (days) | Critical patients % | Drug | Control | Drug vs. control Mortality all patiens | Mortality non-critical | Mortality critical patients | Corticosteroid use |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPACTA N Engl J Med | Jan 2021 | 6 countries, 80% USA, 19 centers | SatO2 < 94% | 389 (249): 128 | Yes | 28 | 26.5% | TCZ 8 mg/Kg (+1) | PLA | 10% vs. 9% | Not shown | Not shown | 80% drug 87% PLA |
| CORIMUNO-19 JAMA Intern Med | Jan 2021 | France, 9 centers | Severe pneumonia = 3 L/min O2 | 131 (64): 67 | No | 28 (median) | 0.0% | TCZ 8 mg/Kg days 1-(3) | ST | 11% vs. 16% | 11% vs. 16% | Not included | 33% drug 61% ST |
| COVACTA MedRxiv | Sep 2020 | North America/Europe | Severe/critical PaO2/FiO2 < 300 mmHg/SatO2 < 93% | 452 (294:144) | Yes | 28 | 37.0% a 68.0% | TCZ 8 mm/Kg* | PLA | 20% vs. 19% | 10% vs. 4% | 24% vs. 28% | 36% drug 54% PLA |
| RCT-TCZ-COVID-19 JAMA Intern Med | Jan 2021 | Italy, 11 centers | Inflammatory PaO2/FiO2 200-300 mmHg | 126 (60:66) | No | 30 | 0.0% | TCZ 8 mg/Kg* (+1 in 12 h) | ST | 3% vs. 2% | 3% vs. 2% | Not included | 0% drug 0% ST |
| BACC Bay N Engl J Med | Dec 2020 | USA, 8 centers | Inflammatory, any risk factor | 243 (161:82) | Yes | 28 | 20.0% | TCZ 8 mg/Kg* | PLA | 6% vs. 4% | Not shown | Not shown | 11% drug 6% PLA |
| REMAP-CAP MedRxiv | Ene 2021 | Europe/North America/Australia | Critical respiratory or cardiovascular support | 803 (401:402) | No | 21 | 100.0% | TCZ 8 mg/Kg (+1 in 12/24 h)* or SRL 400 mg§ | ST | 37% vs. 36% | Not included | 27% vs. 36% | 85%, not specified by arm |
| TOCIBRAS BMJ | Jan 2021 | Brasil, 9 centers | Inflammatory, severe/critical with O2/VM | 129 (65:64) | No | 15 | 83.7% | TCZ 8 mg/Kg* | ST | 17% vs. 9% | 27% vs. 30% | 20% vs. 6% | 84% drug 89% ST |
| RECOVERY MedRxiv (2nd random.) | Jan 2021 | UK, 31 centers | Inflammatory, Sat O2 < 92% | 5,116 (2,022: 2,094) | No | 28 | 45.4% | TCZ 8 mg/Kg** (+1 in 12/24 h) | ST | 29% vs. 33% | 19% vs. 22% | 29% vs. 42% | 82% drug 82% ST 2% PREV# |
MV: mechanical ventilation; PLA: placebo; SRL: sarilumab; ST: standard therapy; TCZ: tocilizumab.
Mortality data from individual studies and meta-analysis. Randomized controlled trials of interleukin-6 inhibitors in hospitalized patients with COVID-19; included critical (with high-flow oxygen or invasive/no invasive mechanical ventilation) and non-critical patients
| Population | All patients | Non-critical patients | Critical patients | Days of symptoms (median) | Days from admission (median) | |||
|---|---|---|---|---|---|---|---|---|
| Reference | Mortality data Drug vs. control | OR (95% CI) | Mortality data Drug vs. control | OR (95% CI) | Mortality data Drug vs. control | OR (95% CI) | ||
| EMPACTA N Engl J Med | 26/249 vs. 11/128 | 1.24 (0.59-2.60) | Not shown | Not shown | Not shown | Not shown | 8.0-8.0 | 6.0-7.5 |
| CORIMUNO 19 JAMA Intern Med | 7/64 vs. 8/67 | 0.63 (0.23-1.73) | 7/64 vs. 8/67 | 0.91 (0.31-2.66) | Not included | Not included | 10-10 | 1-1 |
| COVACTA N Engl J Med | 58/294 vs. 28/144 | 1.02 (0.62-1.68) | 9/87 vs. 2/50 | 2.77 (0.57-13.36) | 49/207 vs. 26/94 | 0.81 (0.47-1.41) | 11-10 | Not shown |
| RCT-TCZ-COVID-19 JAMA Intern Med | 2/60 vs. 1/63 | 2.24 (0.20-25.37) | 2/60 vs. 1/63 | 2.24 (0.20-25.37) | Not included | Not included | 7-8 | 2-2 |
| BACC Bay N Engl J Med | 9/161 vs. 3/82 | 1.56 (0.41-5.92) | Not shown | Not shown | Not shown | Not shown | 9-10 | Not shown |
| REMAP-CAP N Engl J Med | 108/395 vs. 142/397 | 0.68 (0.50-0.91) | Not included | Not included | 108/395 vs. 142/397 | 0.68 (0.50-0.91) | N/D | 1.2/1,4#-1.2 |
| TOCIBRAS BMJ | 11/65 vs. 6/64 | 1.97 (0.68-5.69) | 3/11 vs. 3/10 | 0.88 (0.13-5.82) | 11/54 vs. 3/54 | 4.35 (1.14-16.60) | 10-9.5 (media) | Not shown |
| RECOVERY MedRxiv | 596/2,022 vs. 694/2,094 | 0.84 (0.74-0.96) | 175/935 vs. 202/933 | 0.83 (0.66-1.05) | 421/1,087 vs. 492/1,161 | 0.86 (0.72-1.02) | 9-10 | 2-2 |
| Primary meta-analysis | 817/3,310 vs. 893/3,039 | 0.85 (0.74-0.99) I2 = 7% | 196/1,157 vs. 216/1,123 | 0.86 (0.69-1.07) I2 = 0% | 589/1,753 vs. 663/1,706 | 0.85 (0.62-1.17) I2 = 62% | − | − |
| Sensitivity M-A (exc. RECOVERY) | 221/1,288 vs. 199/945 | 0.99 (0.70-1.27) I2 = 20% | 21/222 vs. 14/190 | 1.27 (0.59-2.75) I2 = 0% | − | − | − | − |
| Sensitivity M-A (exc.RECOVERY/TOCIBRAS) | − | − | − | − | 157/602 vs. 168/491 | 0.70 (0.54-0.91) I2 = 0% | − | − |
M-A: meta-analysis; OR (95%CI): odds ratio (95% confidence interval).
Meta-analysis of mortality for interleukin-6-inhibitors in hospitalized patients with COVID-19. Randomized effects model (DerSimonian-Laird) meta-analysis. A) Forest plot of odds ratios (CI95%). B) Funnel plot. Open circles show missing studies estimated with the trim-and-fill method.
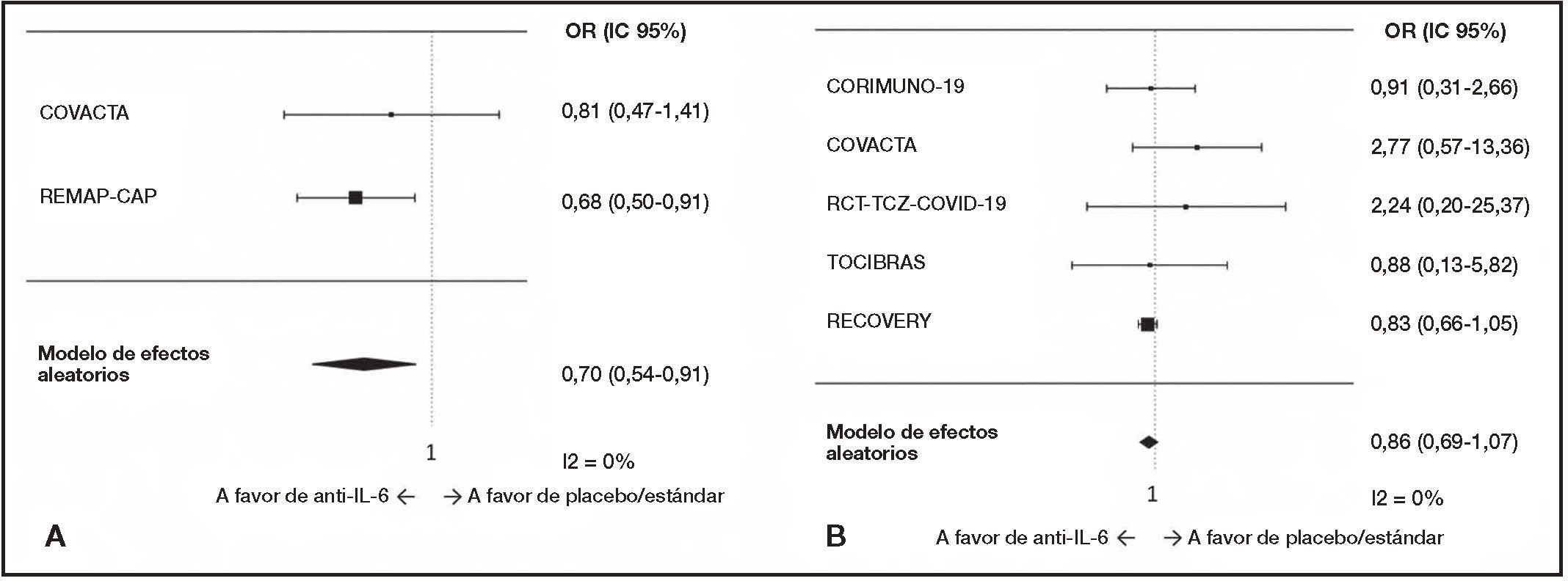
Six trials included ICU/critical patients. The REMAP-CAP study included critical patients only6. EMPACTA12 and BACC-Bay15 did not show any subgroup analysis by respiratory support at baseline and were excluded from the separate analysis. The meta-analysis of non-critical patients showed low heterogeneity (I2 = 0%), with five RCTs and 2,280 patients (Table 2 and Figure 3). In this subset, no benefit was found, including the RECOVERY trial or not, with an odds ratio of 0.86 (95%CI 0.69-1.07), and 1.27 (95%CI 0.59-2.75) excluding the RECOVERY study.
Forest plot of mortality for interleukin-6 Inhibitors In hospitalized COVID-19 patients. A) Critical (with high-flow oxygen or invasive/no Invasive mechanical ventilation at baseline); sensitivity meta-analysis excluding two trials because of lack of similarity (RECOVERY) or homogeneity (TOCIBRAS). B) Non-critical (with non-high-flow/oxygen or without respiratory support at baseline) COVID-19 patients. Randomized effects model (DerSimonian-Laird).
For critical patients, with four RCTs and 3,459 patients included, a large heterogeneity was found (I2 = 62%), with an odds ratio of 0.85 (95%CI 0.62-1.17), as seen in table 2 and figure 3. The TOCIBRAS trial, with only 54 critical patients by arm, a pre-specified time frame of 15-days for outcome evaluation and a low mortality rate in the control group16 was identified as the only source of heterogeneity. In a sensitivity analysis excluding TOCIBRAS and RECOVERY trials because of heterogeneity and lack of similarity, respectively, a low heterogeneity was found (I2 = 0%) with an OR of 0.70 (95%CI 0.54-0.91).
No significant interaction was found among critical and non-critical patients when all trials were included. Excluding RECOVERY from both subgroups and TOCIBRAS from the subgroup of critical patients, interaction p was 0.15 (non-significant).
Only one trial (RECOVERY) showed separate analysis for patients with or without concomitant steroid use with a rate ratio of 0.84 (95%CI 0.75-0.93) with steroid use and 1.16 (95%CI 0.91-1.48) without steroids7. An interaction p of 0.01 was estimated, indicating that the benefit could be selective to patients with concomitant use of tocilizumab and corticosteroids.
DiscussionAfter this systematic review and meta-analysis, with eight RCTs and 6,049 patients included, interleukine-6 inhibitors showed a statistically significant benefit in mortality, but the differences among the patients included in the different studies complicate a general recommendation. It seems necessary to identify what type of patients could benefit from the intervention. The meta-analysis of subgroups with critical and non-critical patients showed no significant interaction, and so it is possible that the differences were due to chance or lack of statistic power.
The RECOVERY study showed a rare scenario nowadays, with patients not receiving steroids during the 48 hours after hospital admission, in a first randomization to other treatments in the study and then receiving tocilizumab (or not) with steroids. Further, the profile of patients included in this second randomization were patients with a clear inflammatory pattern (basal C-reactive protein ≥ 75 mg/L) and markedly hypoxemic (Sat O2 < 92%). This selection of patients and the clinical setting could explain the difference in the results compared to previous studies. Therefore, the exclusion in a meta-analysis such as the one elaborated in this publication would be justified.
Two trials, CORIMUNO-1915 and COVACTA4, were severely unbalanced for corticosteroid use, with an increased use in control arm. Due to the known favourable effect of dexamethasone over survival5, this could bias the results against the treatment.
The REMAP-CAP study included specifically ICU-admitted patients, but only 29% of them had mechanical ventilation at baseline6. Although it is a multicentric and multinational study, the relatively low percentage of patients with mechanical ventilation at ICU wards could differ from the clinical practice in other centers or situations. These centers were previously working together as an adaptive platform for the research of several interventions for community-acquired pneumonia at ICU19. A time frame of 48 hours or less from hospital to ICU admission was an eligibility criterion20. So, the time from hospital admission for included patients (1.2 days) was the same that time from ICU admission (14 h) at baseline, showing that they were recently hospital-admitted patients directly referred to the ICU.
The REMAP-CAP study tested two monoclonal antibodies against interleukin-6 in the intervention arm, but only 12.0% of them were prescribed sarilumab; the rest used tocilizumab. The study is under-powered to test differences between them and its design does not address that comparison or a separate analysis, as the two drugs were randomized together in the same arm and could have been selected at different centers and for patients with different characteristics.
In January 2021, after the release of the positive results from the REMAP-CAP trial, NHS guidelines for COVID-19 treatments were updated with an interim statement including interleukin-6 inhibitors use in critical patients21. Based on the scant evidence, the UK recommendations on tocilizumab use state that dexamethasone must be concomitantly or previously used, which is justified to prevent tocilizumab from being prescribed as an alternative to dexamethasone, given that benefit of dexamethasone is better established.
The primary meta-analysis in the whole population showed a protective effect of tocilizumab. A general recommendation about tocilizumab use would be premature, because a sensitivity analysis excluding the RECOVERY trial, a large study including tocilizumab in a second randomization, did not show a consistent effect. The separate analysis in non-critical patients did not show a benefit in mortality, although the precision of the result was low. In critical patients, the results were heterogeneous, but a sensitivity analysis excluding the RECOVERY and TOCIBRAS trial because of dissimilarity and heterogeneity showed a possible protective effect.
It may be surprising that the global meta-analysis showed homogeneity, while the partial subanalysis in critically ill patients did not. However, it must be taken into account that the separate analysis of critical/non-critical patients does not analyze complete studies, but rather groups the critical/non-critical patients from the various studies. The few critically ill patients in the TOCIBRAS study who presented very high mortality showed a markedly different result than in the rest of the studies.
Positive results for tocilizumab are mainly caused by two RCTs with different scenarios, but they are similar in concomitant use of steroids and very-high mortality in critical patients (36% in REMAP-CAP and 42% in RECOVERY). More studies focused on critical patients are needed to address the specific effectiveness of interleukin-6 inhibitors in this setting.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Early Access date (05/03/2022).