To determine the value contribution of cabotegravir + rilpivirine, the first injectable every two months long-acting antiretroviral regimen, using multi-criteria decision analysis.
MethodThe study was developed in two phases. After a small pilot, a field work study with a larger number of multidisciplinary experts was carried out. Seven single-tablet regimens, currently recommended by the GeSIDA guidelines, were selected as comparators. EVIDEM methodology was followed, with a framework composed by 12 quantitative and 5 contextual criteria. Mean and standard deviations were calculated for quantitative criteria (1 to 5 scale; comparative criteria -5 to +5), whereas qualitative criteria were analyzed as percentages of experts that considered a positive, neutral or negative impact for the National Health System. Results: 35 experts participated in the study. Human immunodeficiency virus-1 infection was considered severe (mean ± standard deviation: 3.0 ± 1.0), with moderate size of affected population (2.7 ± 1.2) and unmet needs (2.8 ± 1.0). Minimal differences were found in comparative efficacy/effectiveness (0.1 ± 0.5), safety/tolerability (-0.5 ± 0.7), and cost criteria: cost of the intervention (0.5 ± 2.0), other medical costs (0.2 ± 1.8) and non-medical/indirect costs (0.5 ± 1.6). Experts perceived an improvement with cabotegravir + rilpivirine long-acting, compared to current daily oral single-tablet regimens, in patient-reported outcomes (2.7 ± 1.4). Therapeutic benefit of the long-acting regimen was considered moderate-to-high (3.5 ± 1.2). Experts considered the evidence provided by cabotegravir + rilpivirine long-actingrobust (4.3 ± 0.8), with elevated consensus on its future recommendation in guidelines (3.2 ± 1.0). In contextual criteria, most experts considered positive the impact on population priorities and access (91%), common goal and specific interests (63%) and political, historical, and cultural context criteria (60%). Impact was neutral in system capacity and appropriate use (40%), and opportunity costs and affordability criteria (51%). Result of the weighted global value contribution of cabotegravir + rilpivirine long-acting was 0.34 (-1 to +1 scale), with Patient Reported Outcomes comparative criterion bringing the highest added value.
ConclusionsCabotegravir + rilpivirine long-acting provides added value contribution to human immunodeficiency virus-1 management in Spain compared to daily oral single-tablet regimens. Patient Reported Outcomes and therapeutic benefit of cabotegravir + rilpivirine long-acting were highly valued by experts, as the expected benefit in adherence and stigma-related issues would improve overall quality of life for people living with human immunodeficiency virus-1.
Determinar la contribución de valor de cabotegravir + rilpivirina, el primer tratamiento antirretroviral inyectable de acción prolongada, utilizando metodología de análisis de decisión multicriterio.
MétodoEl estudio se desarrolló en dos fases: una prueba piloto y una fase de extensión, con un grupo multidisciplinar más grande. Se seleccionaron siete regímenes de comprimido único orales diarios recomendados en las guías GeSIDA como comparadores. Se utilizó el marco EVIDEM, compuesto por 12 criterios cuantitativos y 5 contextuales. Los criterios cuantitativos se analizaron calculando la media y desviación estándar, y los cualitativos se analizaron mediante el porcentaje de expertos que consideraron el impacto positivo, neutro o negativo para el Sistema Nacional de Salud.
ResultadosUn total de 35 expertos participaron en el estudio. La infección por virus de la inmunodeficiencia humana 1 se consideró grave (media ± desviación estándar: 3,0 ± 1,0), con un tamaño de población afectada (2,7 ± 1,2) y unas necesidades no cubiertas (2,8 ± 1,0) moderadas. Las diferencias fueron mínimas en los criterios comparativos de eficacia/efectividad (0,1 ± 0,5), seguridad/tolerabilidad (–0,5 ± 0,7) y coste: coste del tratamiento (0,5 ± 2,0), otros costes médicos (0,2 ± 1,8) y costes no-médicos/indirectos (0,5 ± 1,6). Los expertos observaron una mejora con cabotegravir + rilpivirina de acción prolongada en los resultados reportados por los pacientes (2,7 ± 1,4). El beneficio terapéutico (3,5 ± 1,2) se consideró moderado-alto. La evidencia de cabotegravir + rilpivirina de acción prolongada fue considerada robusta (4,3 ± 0,8), con elevado consenso sobre su futura recomendación en las guías (3,2 ± 1,0). En los criterios contextuales, el impacto fue positivo en los criterios de prioridades de acceso (91%), objetivo común (63%) y contexto político (60%). El impacto fue neutro en la capacidad del sistema (40%) y los costes de oportunidad (51%). El resultado promedio de la contribución del valor global de cabotegravir + rilpivirina de acción prolongada fue de 0,34 (escala de -1 a +1), siendo el criterio de resultados reportados por el paciente el que proporcionó la mayor contribución de valor (0,04).
ConclusionesCabotegravir + rilpivirina de acción prolongada aporta un valor añadido en el manejo del virus de la inmunodeficiencia humana 1 en España en comparación con los regímenes de comprimido único utilizados actualmente. Los expertos valoraron positivamente los resultados reportados por los pacientes y el beneficio terapéutico de cabotegravir + rilpivirina de acción prolongada, considerando que el beneficio esperado en la adherencia y los problemas relacionados con el estigma produciría una mejora en la calidad de vida de las personas con virus de la inmunodeficiencia humana 1.
The significance and the consequences of living with human immunodeficiency virus-1 (HIV-1) remain serious public health problems in Spain1. According to the latest estimates, 151,387 people in our country live with HIV2, and the annual incidence of new HIV diagnoses has remained above 3,500 cases in the last 10 years3.
As a result of the advances in antiretroviral treatments (ARTs), HIV has become a chronic disease, with the life expectancy of people living with HIV (PLHIVPLHIV) nearing that of the general population4,5. Consequently, PLHIV are also at a higher risk of experiencing age-related comorbidities6,7. Clinical management of HIV-1 infection should therefore not focus solely on virologic suppression but also on the management of comorbidities (e.g., hypertension, myocardial infarction or impaired renal function), the use of concomitant treatments (including long-term drug-drug interactions), treatment-related toxicity and la increasing the patients’ quality of life6–9.
Current recommendations of the GeSIDA guidelines regarding ART in Spain are mainly based on once-daily single-tablet oral regimens (STRs)10. Currently, ART constitutes a chronic lifelong treatment, and innovative antiretroviral drugs with less frequent dosing regimens and alternative routes of administration, such as injectables, offer these patients novel therapeutic options with the potential to exert a positive impact on convenience and quality of life11.
Cabotegravir + rilpivirine long-acting (CAB+RPV LA) is an innovative ART that has recently been approved in Europe for the treatment of virologically suppressed adults, being the first long-acting injectable every two months for PLHIV12.
The purpose of this study was to determine the value contribution of the long-acting CAB+RPV regimen as compared with once-daily oral SRTs currently recommended in Spain, using multiple-criteria decision analysis (MCDA). MCDA is a healthcare decision-making support tool that makes it possible for the assessment of new drugs to go beyond the classical efficacy, safety and cost criteria13.
MethodsThe present study used the reflective MCDA EVIDEM methodology14, validated by different studies, to determine the value contribution of medications and decision-making processes in Spain15–17. The EVIDEM framework is meant to encourage a reflective and structured multidisciplinary discussion through a set of quantitative and qualitative criteria that underpin the ethical foundations of any decision-making process. It is structured into five quantitative domains with 12 criteria, and 2 contextual domains with 5 criteria (Table 1).
Criteria under the MCDA EVIDEM framework examined in this study
| Quantitative criteria of the MCDA EVIDEM framework |
|
|
|
|
|
| Contextual criteria of the MCDA EVIDEM framework |
|
|
Direct comparisons with the treatment cost criteria were not possible as CAB+RPV long-acting regimen had not been approved in Spain at the time the study was carried out, which means that its cost was not available. Instead, a calculation was made of the mean price of the different therapeutic alternatives, and experts were asked whether the price reference for CAB+RPV long-acting regimen should be at a higher, lower or similar level to those of the alternatives. The experts consequently rated the treatment cost on the basis of the selected hypothetic price reference.
The value contribution of CAB+RPV long-acting was determined in comparison seven daily oral SRTs currently recommended in Spain by the GeSIDA guidelines as the latter are widely used regimens known for promoting therapeutic adherence and quality of life. These regimens were: dolutegravir/abacavir/lamivudine, elvitegravir/cobicistat/emtricitabine/ tenofovir alafenamide, darunavir/cobicistat + emtricitabine/tenofovir alafenamide, rilpivirine/emtricitabine/tenofovir alafenamide, bictegravir/emtricitabine/tenofovir alafenamide, dolutegravir/rilpivirine and dolutegravir/ lamivudine10.
A review of the literature was carried out using the EVIDEM methodology so as to compile relevant information for each one of the criteria included in the framework, creating an evidence matrix.
The evidence was obtained from biomedical databases (PubMed/ Medline), the GeSIDA guidelines10, nationwide and regional HIV/AIDS strategic plans18, and the HIV Monitoring Unit of the Spanish Health Ministry2,3. Clinical data was obtained from phase III clinical trials on CAB+RPV long-acting regimen19–22 and on the seven alternative STRs, in virologically suppressed PLHIV (included as supplementary material), from the SmPCs and European public assessment reports (EPARs) of the European Medicines Agency (EMA), and on the therapeutic positioning reports of the Spanish Medicines and Medical Devices Agency (AEMPS).
The study was made up of two phases. The first phase consisted in a pilot study with a limited number of experts who rated the evidence matrix and conducted a reflective debate on the methodology and the results obtained. This was followed by an extension phase where a larger group of experts rates the evidence matrix validated during the first phase. The feedback of a larger number of experts added robustness to the results.
The experts who participated in the two phases of the process made up a multidisciplinary group that comprised HIV specialists, hospital pharmacists, healthcare evaluators/managers, nurses and patient association representatives. The idea was to cover the widest possible spectrum of agents involved in the management of the disease and in the evaluation of medications from a social perspective. The panel was selected based on the individual's experience.
The expert panel rated the MCDA evidence matrix based on the information presented to them. Scores for non-comparative criteria could range between 0 and +5, and between –5 and +5 for comparative criteria. Contextual criteria were evaluated depending on whether they exerted a positive, neutral or negative impact on the Spanish Health System.
A two-way ANOVA test was carried out to analyze the differences between the scores assigned in the two phases of the study. All the quantitative criteria were included in the analysis, except for the intervention cost (given that the score was contingent on a qualitative assessment). The Šidák test for multiple comparisons was performed to evaluate the differences in the mean scores assigned to each criterion in the two phases.
The scores of the quantitative criteria were presented as mean, standard deviation (SD) and range. The results of the seven comparisons was reflected under the comparative results domain; the mean value was also calculated.
The value contribution of CAB+RPV long-acting regimen was determined based on the weighting of the scores assigned by 98 Spanish regional evaluators23. The value contribution was calculated by multiplying the weighting by standardized scores. The overall value contribution of CAB+RPV long-acting regimen was obtained by adding up the individual value contribution of each quantitative criterion.
The scores of the qualitative criteria were shown on a numerical scale. They ranged from –1, 0 to +1 when the impact was considered negative, neutral or positive, respectively, and the results were adjusted so that they could be presented in terms of the percentage of experts who favored each option.
ResultsThe scores obtained in the two phases of the study were first analyzed separately and were subsequently grouped together for the final analysis, as no statistically significant differences were found between the mean scores of the two phases for any of the criteria analyzed (two-way ANOVA test: p > 0.05; Šidák test: p > 0.05 for all the compared criteria).
A total of 35 experts participated in the study: 10 in the pilot phase and 25 in the extension phase. The final panel included 8 HIV specialists, 6 hospital pharmacists, 6 healthcare evaluators/managers, 8 nurses and 7 patient association representatives. In the second phase, one of the experts (patient representative) preferred not to assign points under the comparative results due to their lack of experience in evaluating clinical data.
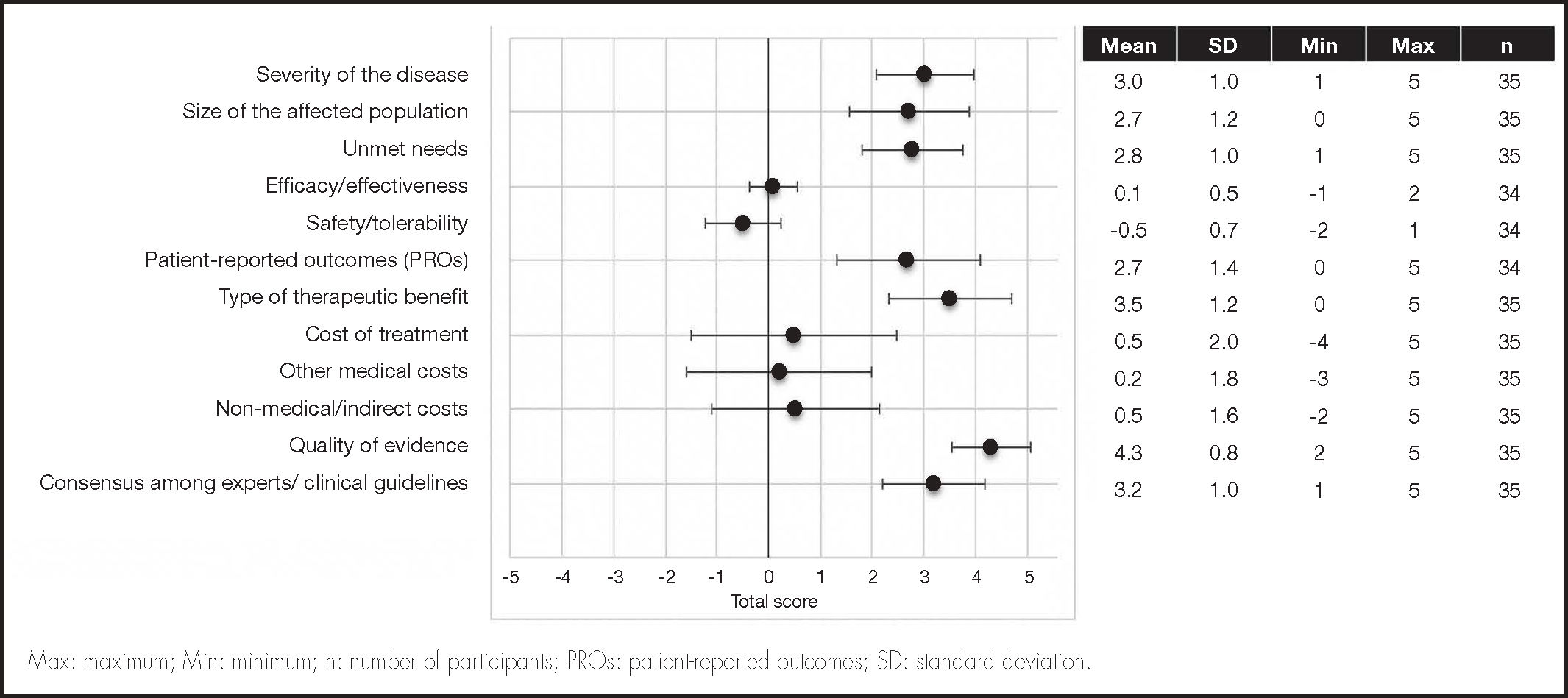
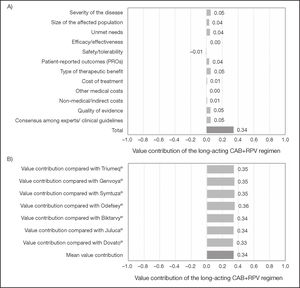
The results are shown in Figure 1. HIV-1 infection is still considered as a severe condition (mean ± SD: 3.0 ± 1.0), as it still requires chronic treatment and PLHIV usually develop more comorbidities than the general population. The size of the affected population (2.7 ± 1.0) and the number of unmet needs (2.8 ± 1.0) were considered of moderate significance, mainly due to the lack of a curative treatment and the sizable number of problems faced by patients, including the social stigma and the neglect of their mental and emotional health problems.
When making a comparison with the seven once-daily oral SRT alternatives, experts perceived the efficacy/effectiveness of the long-acting CAB+RPV regimen as non-inferior (0.1 ± 0.5). Scores for safety/tolerability, though similar, were somewhat lower (–0.5 ± 0.7) due to the presence of advance reactions at the injection site. In the experts’ opinion, CAB+RPV long-acting regimen presented with a superior patient-reported outcome (PRO) profile than the seven oral STR alternatives (2.7 ± 1.4). These results were based on the patients’ preference for the long-acting CAB+RPV regimen and their higher level of satisfaction with it. Experts believed that, in spite of the bias induced by the fact that the treatment preference and satisfaction data were gathered from patients included in the clinical trial, those were important aspects that could favor the prescription of CAB+RPV long-acting regimen.
Experts considered that the introduction of CAB+RPV long-acting regimen could have a neutral effect on the cost of treatment as compared with the seven oral STR alternatives (0.5 ± 2.0). The impact of CAB+RPV long-acting regimen on the other medical cost and medical/indirect cost criteria was also neutral (0.2 ± 1.8 and 0.5 ± 1.6, respectively).
Experts considered that the potential therapeutic benefit was moderatehigh (3.3 ± 1.2), particularly in patients with low levels of adherence or who can be highly affected by the stigma associated with HIV. The quality of the available evidence on CAB+RPV long-acting regimen was considered high, as was that of the seven daily oral STR alternatives (4.3 ± 0.8), there being a high level of consensus that CAB+RPV long-acting regimen will be recommended by the Spanish clinical guidelines (3.2 ± 1.0).
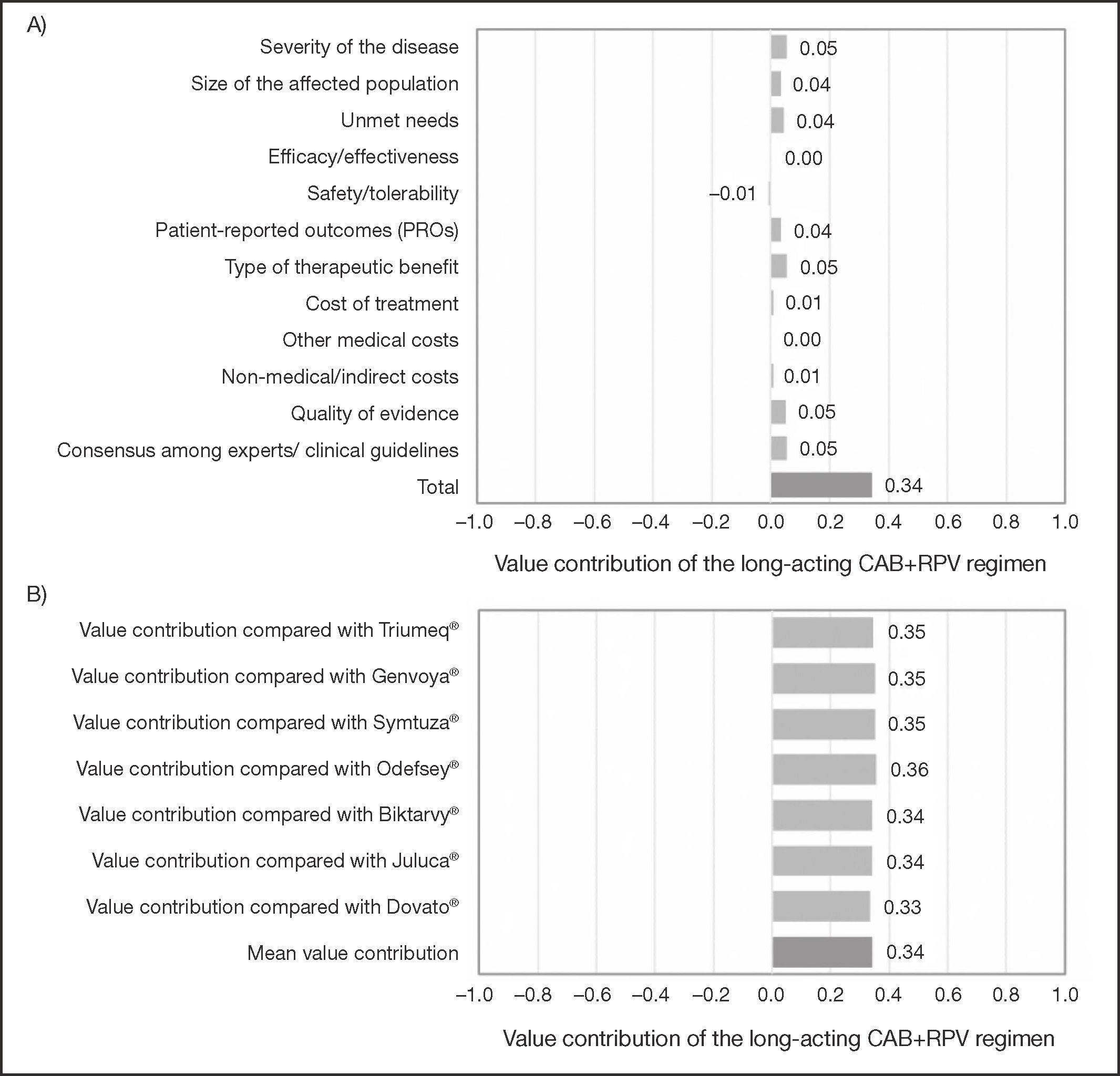
The scores assigned to the different criteria were weighted in order to estimate the overall value contribution of CAB+RPV long-acting regimen as compared with the seven daily oral STR alternatives (Figure 2A). The result was 0.34 (scale ranging from –1 to +1). As regards the disease criteria, the greatest overall value contributions were made by severity of the disease, the type of therapeutic benefit obtained, and the experts’/clinical guidelines consensus, with a score of 0.05 each. For the comparative criteria, added value was centered on PROs, with a score of 0.04. The value contribution of CAB+RPV long-acting regimen as compared with each individual STR was similar to its overall contribution (Figure 2B).
Value contribution of CAB+RPV long-acting regimen based on quantitative criteria. A) Overall weighted value contribution of the long-acting CAB+RPV regimen as compared with the seven alternative STRs. B) Individual weighted value contribution of the long-acting CAB+RPV regimen as compared each one of the seven alternative STRs.
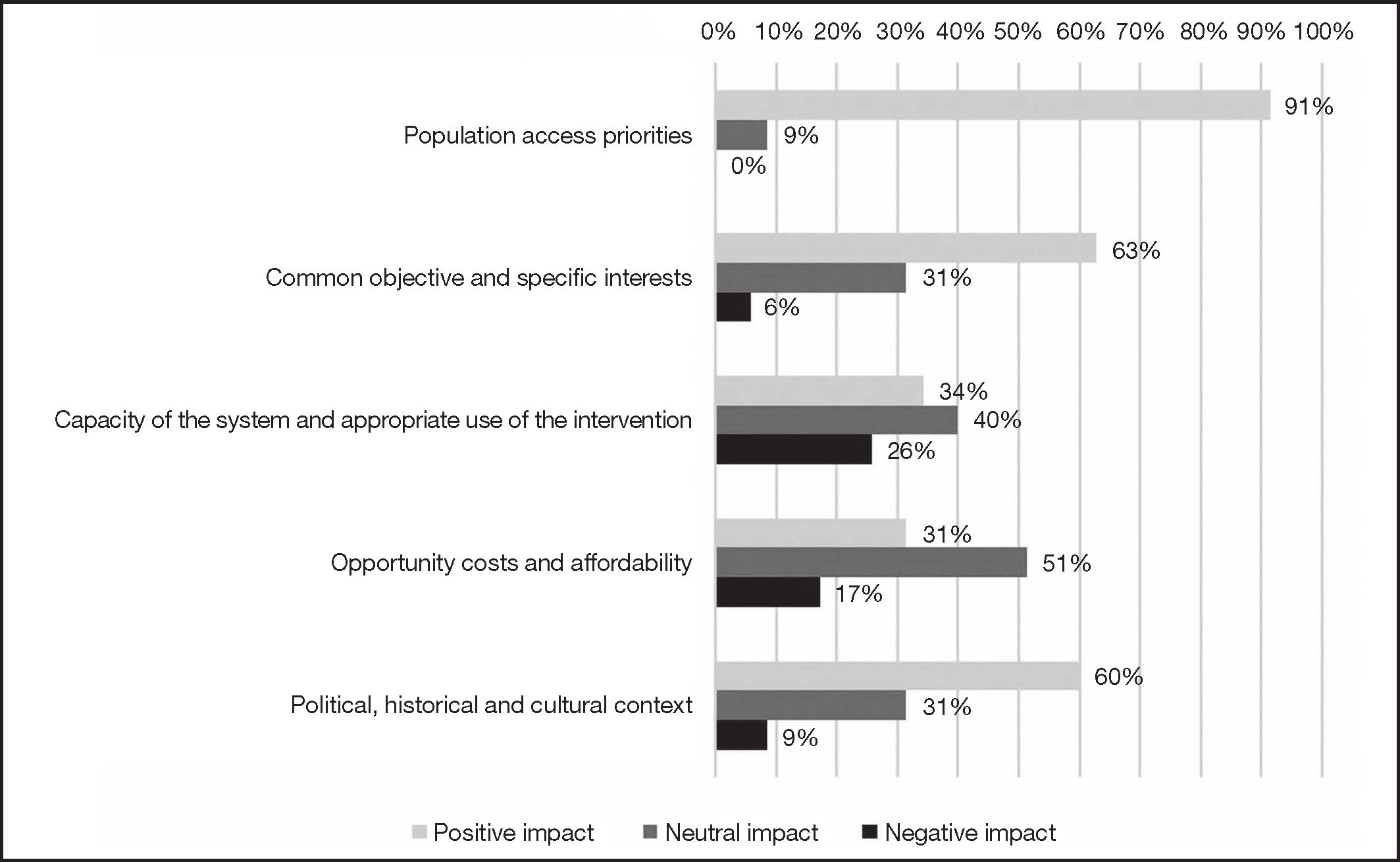
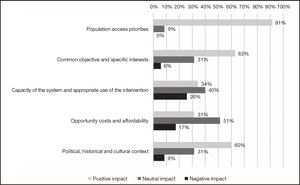
As far as the contextual criteria are concerned (Figure 3), 91% of experts thought that CAB+RPV long-acting regimen was aligned with the interests and objectives of the Spanish Health System as it could contribute to reducing the stigma and discrimination associated with HIV, improving the privacy of some patients, and promoting therapeutic adherence. Most experts (63%) also agreed that no significant obstacles were likely to hinder PLHIV's access to treatment with CAB+RPV long-acting regimen.
The majority of experts considered that the Spanish Health System was prepared to implement the long-acting CAB+RPV regimen and ensure its appropriate use. The new regimen's impact was deemed positive by 34% of respondents and as neutral by 40%. They also agreed that no significant opportunity costs or affordability impacts were likely (51% anticipated a neutral impact) and that the political, historical and cultural would have a positive impact on the incorporation of CAB+RPV long-acting regimen, as the measures geared towards improving the health services offered to PLHIV enjoyed the required institutional and political support (60% positive impact).
DiscussionThe purpose of this study was to determine the value contribution of CAB+RPV long-acting regimen, the first every two months long-acting injectable treatment, as compared with oral single-tablet antiretroviral regimens currently recommended for the treatment of HIV-1 in Spain, through a reflective MCDA carried out by a multidisciplinary expert panel. An extension phase with a greater number of experts was added to increase the reliability (test-retest approach) and robustness of the results. No statistically significant differences were found in any of the criteria analyzed and the experts’ reflections were consistent across the two phases. The reflective MCDA debate during the pilot phase provided essential information to better understand and discuss the results obtained. This allowed a holistic determination of the value of CAB+RPV long-acting regimen against the specific background of its role in the Spanish context.
A wide-ranging expert panel, which included the main actors involved in the management of HIV and in the social-based evaluation of medication regimes, found that CAB+RPV long-acting regimen would make a valuable contribution to the treatment of HIV-1, as compared with the oral STR alternatives recommended in Spain. The criteria that made the greatest contribution to the overall value of CAB+RPV long-acting regimen were PROs, with the patients’ preference for the long-acting injectable regimen vs. the daily oral treatment, and their satisfaction with the CAB-RPV treatment demonstrated in clinical trials constituting two aspects likely to improve the patients’ quality of life. Durante the reflective debate, some experts mentioned that the preference and satisfaction results included in the evidence matrix could have been biased in favor of the long-acting regimen as they came from data obtained during the clinical development of the long-acting CAB+RPV treatment19–22. However, it must be taken into consideration that patient preference could also be the main reason for prescribing CAB+RPV long-acting regimen once it becomes available11.
The outcomes of the efficacy/effectiveness, safety/tolerability and cost criteria showed a limited contribution to the overall value, suggesting a high degree of similarity between CAB+RPV long-acting regimen and the seven oral STR alternatives for these criteria. Safety/tolerability comparisons obtained slightly lower scores given the risk of injection site reactions, which are inherent in intramuscular injections and are therefore not observed or reported with the oral STR alternatives. The experts recognized that those adverse events were mild and resolved within days. Although there is at present no single method capable of measuring therapeutic adherence, making it necessary to resort to a combination of different techniques with an individualized and multidisciplinary approach24, the experts pointed out that CAB+RPV long-acting regimen had the potential to improve PLHIV's therapeutic adherence.
As regards the contextual criteria, the experts considered that treatment of HIV-1 with CAB+RPV long-acting regimen was aligned with the priorities of the Spanish Health System and that no significant barriers were to be expected in terms of patients being able to access the new therapy. Most experts considered that hospitals in the Spanish Health System would be capable of managing the drug correctly and that having an alternative ART with an innovative administration route would be positive for all patients.
In Spain, the evaluation of the value contribution of treatments is still largely based on efficacy, safety and cost criteria23, although the use of MCDA in healthcare decision-making has increased in the past few years25. Spanish evaluators and decision-makers have already expressed that the use of MCDA frameworks can be an effective way of evaluating medications and making therapeutic decisions26. For example, Guarga et al. developed an MCDA framework to determine the value of orphan drugs in Catalonia's Health System17. Álvarez-Román et al., for their part, et about determining the value contribution of emicizumab to the treatment of hemophilia A27 and Zozaya et al. sought to establish the value of two biological drugs in the treatment of chronic inflammatory conditions of the skin28. The main limitation of these studies was the reduced number of experts who participated in the drug evaluation process. Jiménez et al. overcame this limitation by increasing the number of participating experts by adding a second online stage29. Conversely, the present study involved a 35-strong multidisciplinary expert panel, following a test-retest approach that made the results obtained more reliable and robust.
This study is however not free of limitations. Firstly, the risk of bias in the process used to select the members of the expert panel cannot be excluded, although strict recruitment and participation criteria were defined. Moreover, the consistency of the results of the two phases of the study would seem to preclude the possibility that the results might have been different with a different expert panel. Another limitation was that the price of CAB+RPV long-acting treatment was unknown at the time the study was carried out. To minimize the impact of this limitation, experts were asked to provide a price range using the mean cost of the seven daily oral STRs as a reference so that they could respond to the criterion relative to the cost of treatment based on their previous response. This means that no significant changes are likely to occur in this criterion once the price is available. Lastly, despite the study involved a larger expert panel than other studies, it could be argued that the number of participating decision-making experts was relatively small. Nonetheless, drug evaluation committees are generally not made up of a high number of evaluators with decision-making responsibilities.
This is the first study to use the MCDA methodology to evaluate the value contribution of CAB+RPV long-acting ART in Spain. Further studies will have to be carried out to extend the use of the MCDA methodology to healthcare decision-making.
From the point of view of Spanish society, CAB+RPV long-acting regimen is regarded as an asset for the management of HIV-1 as compared with its once-daily oral STR alternatives. Experts were positive about the PROs and the therapeutic benefits provided by CAB+RPV long-acting regimen, considering that the benefit expected in terms of therapeutic adherence and the problems related to the stigma of the disease would result in an improvement in the patients’ quality of life. The reflective MCDA methodology has shown itself as a useful tool for highlighting the additional benefits contributed by the first q2m long-acting injectable ART, thereby allowing a more effective decision-making process.
FundingThis study was funded by ViiV Healthcare (study number #214462).
Conflict of interestLaura Amanda Vallejo-Aparicio is employee of and own shares of the GSK Group. Beatriz Hernández-Novoa is employee of and owns shares of ViiV Healthcare. Miguel Ángel Calleja-Hernández and José Manuel Martinez-Sesmero received honoraria for their participation in the study. Xavier Badia is a employee of Omakase Consulting. Omakase Consulting received funds from ViiV Healthcare for the performance of this study.
Presentation at congresses24th National Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology. Online; 5 to 11 June 2021.
Contribution to the scientific literature
This is the first study to use multiple-criteria decision analysis to evaluate the contribution made by the introduction of cabotegravir + rilpivirine, the first long-acting injectable antiretroviral treatment authorized by the Spanish Health System, to antiretroviral treatment in Spain.
We believe the results obtained can be considered significant and may constitute a decision-making tool when treating human immunodeficiency virus-1 patients who are candidates for antiretroviral treatment.
CAB+RPV AP MCDA study group: Agustín Rivero, Antonia Alberta Estévez, Antonio Antela, Antonio Rivero, Boi Ruiz, Carlos Folgueras, Carlos Mur, Daniel Podzamczer, Diego García, Elena Casaus, Emilio Monte, Emma Fernández, Jordi Puig, Jorge Garrido, José Luis Trillo, Juan Miguel Castro, Laura Labajo, Manel Fontanet, Margarita Ramírez, María del Mar Masiá, María Eugenia Negredo, María José Fuster, Marisa Montes, Marta Pastor, Michael Meulbroek, Mireia Santacreu, Pedro Gómez, Pere Ventanyol, Ramón Espacio, Santiago Moreno and Sofía Huete.
Early Access date (06/25/2022).