Older patients are more susceptible to medication use, and physiological changes resulting from aging and organic dysfunctions presented by critically ill patients may alter the pharmacokinetic or pharmacodynamic behavior. Thus, critically ill older people present greater vulnerability to the occurrence of pharmacotherapeutic problems.
ObjectiveTo evaluate pharmacotherapy and the development of potential adverse drug reactions (ADRs) in older patients admitted to an intensive care unit (ICU).
MethodA cohort study was conducted in an ICU for adults of a Brazilian University Hospital during a 12-month period. The patients' pharmacotherapy was evaluated daily, considering the occurrence of ADRs and drug–drug interactions (DDIs), the use of potentially inappropriate medications (PIMs) for older people, and the pharmacotherapy anticholinergic burden (ACB). A trigger tool was used for active search of ADRs, with subsequent causality evaluation. PIM use was evaluated by means of the Beers criteria and the STOPP/START criteria. The ABC scale was employed to estimate ACB. The Micromedex® and Drugs.com® medication databases were employed to evaluate the DDIs.
ResultsThe sample of this study consisted of 41 patients, with a mean age of 66.8 years old (±5.2). The 22 triggers used assisted in identifying 15 potential ADRs, and 26.8% of the patients developed them. The mean estimated ACB score was 3.0 (±1.8), and the patients used 3.1 (±1.4) and 3.3 (±1.6) PIMs according to the Beers and the STOPP criteria, respectively. A total of 672 DDIs were identified, with a mean of 16.8 (±9.5) DDIs/patient during ICU hospitalization. Our findings show an association between occurrence of ADRs in the ICU and polypharmacy (p=.03) and DDIs (p=.007), corroborating efforts for rational medication use as a preventive strategy.
ConclusionsUsing tools to evaluate the pharmacotherapy for older people in intensive care can assist in the recognition and prevention of pharmacotherapeutic problems, with emphasis on the identification of ADRs through the observation of triggers and subsequent causality analysis.
Pacientes de edad avanzada son más susceptibles al uso de medicamentos, y cambios fisiológicos resultantes del envejecimiento y disfunciones orgánicas presentadas por los pacientes críticamente enfermos pueden alterar el comportamiento farmacocinético o farmacodinámico. Por lo tanto, personas de edad avanzada en estado crítico presentan mayor vulnerabilidad a la aparición de problemas farmacoterapéuticos.
ObjetivoEvaluar la farmacoterapia y el desarrollo de posibles reacciones adversas a medicamentos (RAM) en pacientes de edad avanzada ingresados en una unidad de cuidados intensivos (UCI).
MétodoSe realizó un estudio de cohorte en una UCI para adultos de un Hospital Universitario brasileño durante un período de 12 meses. Se evaluó diariamente la farmacoterapia de los pacientes, considerando la aparición de RAM y interacciones medicamentosas (IM), uso de medicamentos potencialmente inapropiados (MPI) para personas de edad avanzada y carga anticolinérgica de la farmacoterapia (CAF). Se utilizó una herramienta tipo “Trigger Tool” para la búsqueda activa de RAM, con posterior evaluación de causalidad. El uso de MPI se evaluó mediante los criterios de Beers y los criterios STOPP/START. Se empleó la escala ABC para estimar la CAF. Las bases de datos de medicamentos Micromedex® y Drugs.com® se emplearon para evaluar las IM.
ResultadosLa muestra de este estudio consistió en 41 pacientes, con edad media de 66,8 años (±5,2). Los 22 triggers utilizados ayudaron a identificar 15 posibles RAM, y el 26,8% de los pacientes las desarrollaron. La puntuación media estimada de la CAF fue de 3,0 (±1,8), y los pacientes utilizaron 3,1 (±1,4) y 3,3 (±1,6) MPI según los criterios de Beers y STOPP, respectivamente. Se identificaron 672 IM, con media de 16,8 (±9,5) IM/paciente durante la hospitalización en UCI. Nuestros hallazgos muestran una asociación entre la aparición de RAM en UCI y polifarmacia (p = 0,03) y IM (p = 0,007), lo que corrobora los esfuerzos para un uso racional de los medicamentos como estrategia preventiva.
ConclusionesEl uso de herramientas para evaluar la farmacoterapia en personas de edad avanzada en UCI puede ayudar el reconocimiento y prevención de problemas farmacoterapéuticos, con énfasis en identificación de RAM a través de observación de triggers y la posterior análisis de causalidad.
Managing critically ill patients involves caring for life-threatening conditions and reducing their risk; however, patients in intensive care units (ICUs) may have pre-existing comorbidities.1,2 Older patients over 60 years old are predominant in terms of ICU admissions due to global population trends.3 Care for these patients must consider age-related physiological changes affecting pharmacokinetics, pharmacodynamics, chronic health problems, and polypharmacy.2
In the older population, careful selection of medications is crucial to prevent pharmacotherapeutic problems. The ICU requires meticulous prescription due to the risk of potentially inappropriate medications (PIMs) for older people.4 Expert reviews and consensus have led to the development of tools such as PIM lists, anticholinergic burden (ACB) scales, and trigger tools for adverse drug reactions (ADRs). Analyzing causality and drug interactions (DIs) databases also assists in evaluating pharmacotherapy and promoting medication safety for older people.
The Beers criteria and STOPP/START (Screening Tool of Older Persons' Prescriptions/Screening Tool to Alert to Right Treatment) are the most significant and established PIM lists.5,6 The Anticholinergic Cognitive Burden scale estimates the ACB of medications and helps evaluate their cognitive effects in older patients, who are more susceptible to developing severe ADRs due to blocking acetylcholine action in the central nervous system (CNS).7
Identifying ADRs in critically ill older patients is challenging due to diverse pharmacotherapeutic management options. Evaluating causality is crucial to confirm the episode and rule out disease processes or physiological status-related events. A higher agreement on the causal link to ADRs strengthens the hypothesis about medication use and adverse events, assisting in therapy optimization and in reducing hospitalization time, recurrence, and iatrogenic complications.8
Detecting DDI is crucial in the pharmacotherapy of critically ill older patients. Concomitant use of medications, food, or diluents may alter the drug effects, potentially amplifying or reducing pharmacological action.8 The objective of this study is to evaluate pharmacotherapy and potential ADR development in older patients admitted to an ICU.
Material and methodsThis is a cohort study conducted in the ICU for adults of the Hospital das Clínicas at the Ribeirão Preto Medical School of the University of São Paulo (HCFMRP-USP); during the study period, the Hospital had 727 beds, 9 of which were in the adult ICU (≥18 years old). The study was approved by the Research Ethics Committee of the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (CAAE 44000715.6.0000.5403).
The patients included were those aged ≥60 years old and hospitalized in the ICU for ≥48 h between September 2016 and September 2017. Individuals whose analysis regarding causality of the ADRs was classified as conditional or doubtful were excluded. The sample was selected by convenience, where participants were chosen based on accessibility and availability for the study, thus facilitating data collection. The participants who developed potential ADRs during the study were allocated to the “Exposed” group, while those who did not were classified as “Unexposed”. The convenience sample was selected by the clinical pharmacist and first author, FAMC. The prescribed pharmacotherapy was evaluated daily, considering occurrence of ADRs, medication errors, use of PIMs, and drug–drug interactions (DDIs).
To ensure quality of the collected data, 2 tools were used to evaluate potential medication errors: Micromedex® Solutions and Drug Interactions CheckerDrugs.com®. These tools were chosen based on their ease of use, scope, and relevant quality scores. Severity of the medication errors was classified as contraindicated, major, moderate, or minor. Only contraindicated interactions and those with major clinical significance were considered in the analysis, as they have higher likelihood of clinical relevance. The criteria used for classification were based on the databases consulted.9,10
The PIMs were assessed via the Beers and STOPP/START criteria. The 2019 Beers criteria5 categorize medications into 5 groups based on their appropriateness for older patients. Category 1 contains PIMs, while Category 2 comprises medications that depend on the underlying disease. Category 3 lists medications that require caution, while Category 4 contains medications with harmful interactions. Lastly, Category 5 contains medications requiring dose adjustments based on kidney function.
The STOPP/START criteria were developed to screen prescriptions for older people, including PIMs (STOPP) and possible omissions in pharmacotherapy that are important for proper treatment (START). They include a total of 114 criteria: 80 for STOPP and 34 for START.6 In this study, only the STOPP criteria were used, divided into A-M sections according to physiological systems or therapeutic classes.
The ACB scale was used to classify medications into 3 categories according to their anticholinergic effect: minimal (score 1), moderate (score 2), and severe (score 3). Considering the sum of the scores of all prescribed medications, a score of 3 or higher is considered clinically significant.11 For the purposes of this study, the highest score obtained during the participant's research follow-up period was considered.
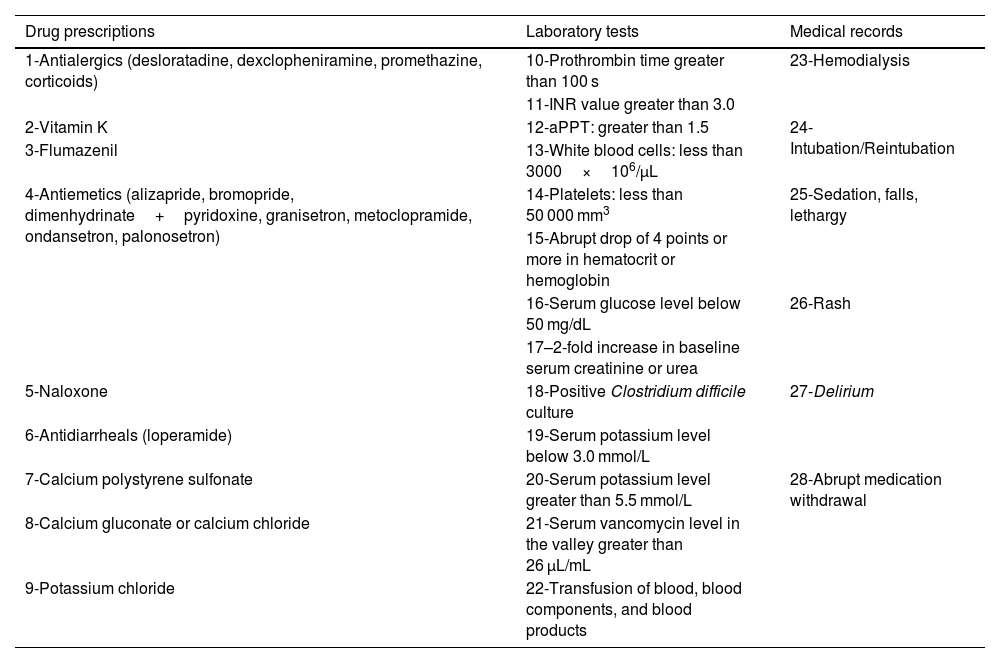
A total of 28 triggers, based on the Institute for Healthcare Improvement Global Trigger Tool for Measuring Adverse Events12 and other triggers, were selected to actively search for ADRs (Table 1). These triggers were complemented by considering standardized medications, laboratory tests, and the routine of the HCFMRP-USP ICU. Triggers were identified in medical prescriptions, laboratory tests, and clinical evolution in the patient's medical record to facilitate the active search for medication-related dysfunctions. Rozich, Haraden, and Resar's13 triggers were also employed.
Triggers for the active search of ADRs in patients admitted to the intensive care unit.
| Drug prescriptions | Laboratory tests | Medical records |
|---|---|---|
| 1-Antialergics (desloratadine, dexclopheniramine, promethazine, corticoids) | 10-Prothrombin time greater than 100 s | 23-Hemodialysis |
| 11-INR value greater than 3.0 | ||
| 2-Vitamin K | 12-aPPT: greater than 1.5 | 24-Intubation/Reintubation |
| 3-Flumazenil | 13-White blood cells: less than 3000×106/μL | |
| 4-Antiemetics (alizapride, bromopride, dimenhydrinate+pyridoxine, granisetron, metoclopramide, ondansetron, palonosetron) | 14-Platelets: less than 50 000 mm3 | 25-Sedation, falls, lethargy |
| 15-Abrupt drop of 4 points or more in hematocrit or hemoglobin | ||
| 16-Serum glucose level below 50 mg/dL | 26-Rash | |
| 17–2-fold increase in baseline serum creatinine or urea | ||
| 5-Naloxone | 18-Positive Clostridium difficile culture | 27-Delirium |
| 6-Antidiarrheals (loperamide) | 19-Serum potassium level below 3.0 mmol/L | |
| 7-Calcium polystyrene sulfonate | 20-Serum potassium level greater than 5.5 mmol/L | 28-Abrupt medication withdrawal |
| 8-Calcium gluconate or calcium chloride | 21-Serum vancomycin level in the valley greater than 26 μL/mL | |
| 9-Potassium chloride | 22-Transfusion of blood, blood components, and blood products |
INR = international normalized ratio; aPTT = activated partial thromboplastin time.
The causality assessment was performed with the aid of the Naranjo Algorithm (NARANJO), the WHO-UMC causality assessment system (WHO-UMC), and the Liverpool ADR Causality Assessment Tool (LCAT). In case of disagreement, agreement was considered when at least 2 of them had concordance using Cohen's Kappa coefficient, through the quality of the classification associated by Landis and Koch (Supplementary table 1). In addition, the ADRs were classified according to criteria proposed by Rawlins and Thompson, which groups them into types of reactions according to their mechanism of action.14 The severity assessment of the ADRs was performed according to the proposal adapted from the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP).15
The associations between the qualitative variables were verified by means of Fisher's Exact test. The linear association between two quantitative variables was determined by Pearson's correlation coefficient (r). To verify the difference between means, the Student's t-test, and the Mann–Whitney test were used. The significance level (α) was fixed at 0.05. The statistical analyses were performed using the Statistical Package for Social Sciences® (SPSS Inc., version 17.1.0).
Results and discussionThe sample of this study consisted of 41 patients. Of these, 21 (51.2%) were male and the mean age of the older patients was 66.8 years old (±5.2). Prior to ICU admission, 41.5% (n=17) of the patients were already hospitalized for a period of ≥14 days, and the admission of 85% (n=35) of the patients was unplanned (not scheduled with less than 12-h notice). Using the trigger tool strategy, 22 triggers assisted in identifying 15 potential ADRs, among which 11 (26.8%) patients developed these potential ADRs and were consequently allocated to the “Exposed” group. Thus, 73.2% of the sample (30 older patients) did not manifest any ADR and comprised the “Unexposed” group.
In the evaluation of agreement between the algorithms for assessing ADR causality, WHO-UMC and LCAT obtained moderate agreement (Kappa=0.43). The association between NARANJO and LCAT showed minimal agreement, followed by NARANJO and WHO-UMC showing moderate agreement, with Kappa values of 0.17 and 0.43, respectively.
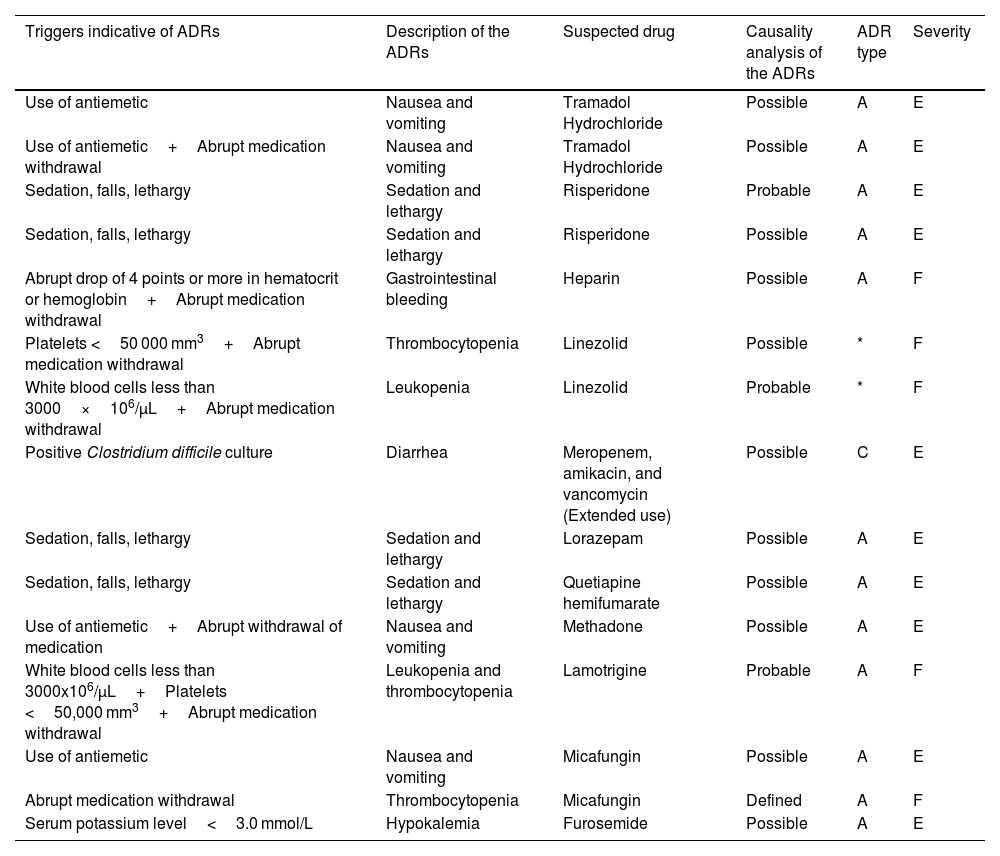
According to the causality assessment performed and considering agreement between at least 2 algorithms, among the 15 suspected ADRs, a classification of definite was assigned to 1 (n=1), probable to 3 (n=3), and possible to 11 (n=11) (Table 2). Prevalence of ADRs with a type A (augmented/dose-related) mechanism of action (80%, n=12) was identified; 1 ADR was identified as type C (chronic/dose-related and time-related) and 2 cases were not classified. ADRs related to linezolid use were not classifiable by type, as they had no relation to the mechanism of action and were described as reactions, although with prolonged use. Linezolid was used within the recommended dose (600 mg every 12 h), for a period shorter than recommended for the treatment (treatment time of 2 and 8 days, with a recommended duration of 10–14 days) and without any cumulative effect, making it impossible to establish a causal link. In the ADR severity assessment, it was identified that 66.7% (n=10) were classified as category E (contributed to or resulted in temporary harm to the patient and required intervention), whereas the remaining 5 were specified as category F (contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization). No permanent damage or life support measures were identified, nor was there any death resulting from ADRs.
Characteristics of the adverse drug reactions in older patients in intensive care.
| Triggers indicative of ADRs | Description of the ADRs | Suspected drug | Causality analysis of the ADRs | ADR type | Severity |
|---|---|---|---|---|---|
| Use of antiemetic | Nausea and vomiting | Tramadol Hydrochloride | Possible | A | E |
| Use of antiemetic+Abrupt medication withdrawal | Nausea and vomiting | Tramadol Hydrochloride | Possible | A | E |
| Sedation, falls, lethargy | Sedation and lethargy | Risperidone | Probable | A | E |
| Sedation, falls, lethargy | Sedation and lethargy | Risperidone | Possible | A | E |
| Abrupt drop of 4 points or more in hematocrit or hemoglobin+Abrupt medication withdrawal | Gastrointestinal bleeding | Heparin | Possible | A | F |
| Platelets <50 000 mm3+Abrupt medication withdrawal | Thrombocytopenia | Linezolid | Possible | * | F |
| White blood cells less than 3000×106/μL+Abrupt medication withdrawal | Leukopenia | Linezolid | Probable | * | F |
| Positive Clostridium difficile culture | Diarrhea | Meropenem, amikacin, and vancomycin (Extended use) | Possible | C | E |
| Sedation, falls, lethargy | Sedation and lethargy | Lorazepam | Possible | A | E |
| Sedation, falls, lethargy | Sedation and lethargy | Quetiapine hemifumarate | Possible | A | E |
| Use of antiemetic+Abrupt withdrawal of medication | Nausea and vomiting | Methadone | Possible | A | E |
| White blood cells less than 3000x106/μL+Platelets <50,000 mm3+Abrupt medication withdrawal | Leukopenia and thrombocytopenia | Lamotrigine | Probable | A | F |
| Use of antiemetic | Nausea and vomiting | Micafungin | Possible | A | E |
| Abrupt medication withdrawal | Thrombocytopenia | Micafungin | Defined | A | F |
| Serum potassium level<3.0 mmol/L | Hypokalemia | Furosemide | Possible | A | E |
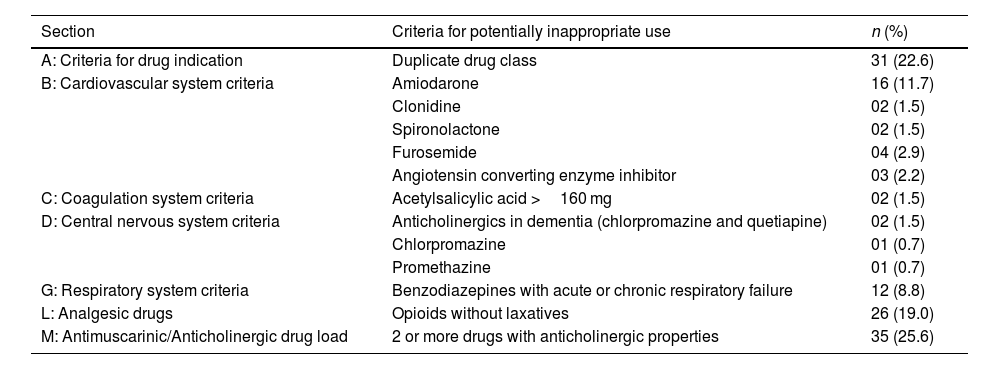
Regarding the evaluation performed using the STOPP criteria (Table 3), the older patients used a mean of 3.3 (±1.6) PIMs, ranging from 1 to 9 medications. Based on the Beers Criteria (Supplementary table 2), the patients used a mean of 3.1 (±1.4) PIMs, varying from 1 to 8. It is noteworthy that, in both analyses performed, only 1 patient did not receive a prescription for PIMs.
Potentially inappropriate medications, according to the STOPP criteria and their respective sections/categorizations, prescribed to older patients in the intensive care unit.
| Section | Criteria for potentially inappropriate use | n (%) |
|---|---|---|
| A: Criteria for drug indication | Duplicate drug class | 31 (22.6) |
| B: Cardiovascular system criteria | Amiodarone | 16 (11.7) |
| Clonidine | 02 (1.5) | |
| Spironolactone | 02 (1.5) | |
| Furosemide | 04 (2.9) | |
| Angiotensin converting enzyme inhibitor | 03 (2.2) | |
| C: Coagulation system criteria | Acetylsalicylic acid >160 mg | 02 (1.5) |
| D: Central nervous system criteria | Anticholinergics in dementia (chlorpromazine and quetiapine) | 02 (1.5) |
| Chlorpromazine | 01 (0.7) | |
| Promethazine | 01 (0.7) | |
| G: Respiratory system criteria | Benzodiazepines with acute or chronic respiratory failure | 12 (8.8) |
| L: Analgesic drugs | Opioids without laxatives | 26 (19.0) |
| M: Antimuscarinic/Anticholinergic drug load | 2 or more drugs with anticholinergic properties | 35 (25.6) |
Only 1 patient did not use medications with anticholinergic properties. The estimated ACB mean score was 3.0 (±1.8), and 53.6% (n = 22) had a clinically relevant score (ACB scale ≥3). Among the drugs with anticholinergic properties (Supplementary table 3), fentanyl was the most prescribed (24.8%), followed by furosemide (14.5%) and hydrocortisone (14.5%).
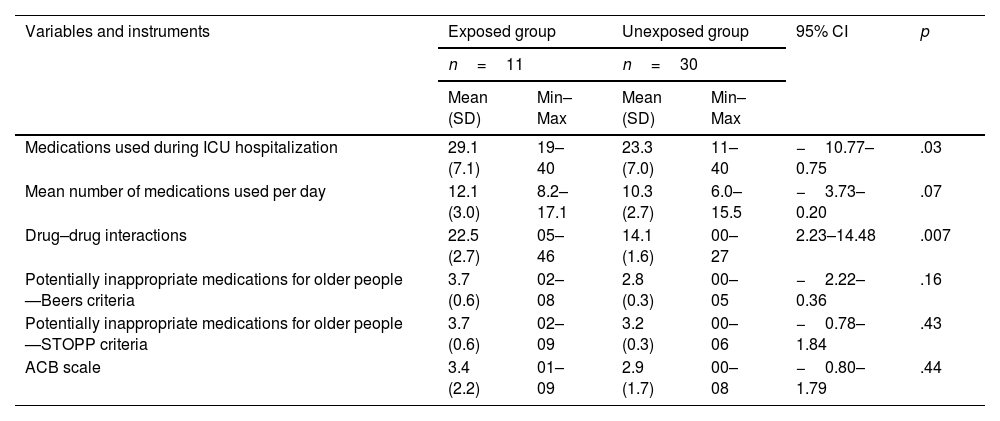
A total of 672 DDIs classified as severe were identified, involving 97.5% (n=40) of the older patients with a mean of 16.8 (±9.5) DDIs per patient during their hospitalization in the ICU. The study revealed various DDIs that including therapeutic combinations common in the ICU and that lead to the exacerbation of CNS depression such as the use of opioids and benzodiazepines, with the interaction between fentanyl and midazolam being the most prevalent9,10 (Supplementary table 4). Fentanyl was the drug with the highest number of DDIs identified, present in 27% of the reported interactions (n=181). In addition, DDIs in which one of the drugs belongs to the opioid class occupy the top 3 positions of the most detected interactions, possibly due to their widespread use in the treatment of pain. It was found that the number of medications used during ICU hospitalization and the number of DDIs were associated with occurrence of ADRs (Table 4).
Pharmacotherapeutic variables and performance of the instruments used in the evaluation of the treatment for older patients in intensive care.
| Variables and instruments | Exposed group | Unexposed group | 95% CI | p | ||
|---|---|---|---|---|---|---|
| n=11 | n=30 | |||||
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | |||
| Medications used during ICU hospitalization | 29.1 (7.1) | 19–40 | 23.3 (7.0) | 11–40 | −10.77–0.75 | .03 |
| Mean number of medications used per day | 12.1 (3.0) | 8.2–17.1 | 10.3 (2.7) | 6.0–15.5 | −3.73–0.20 | .07 |
| Drug–drug interactions | 22.5 (2.7) | 05–46 | 14.1 (1.6) | 00–27 | 2.23–14.48 | .007 |
| Potentially inappropriate medications for older people—Beers criteria | 3.7 (0.6) | 02–08 | 2.8 (0.3) | 00–05 | −2.22–0.36 | .16 |
| Potentially inappropriate medications for older people—STOPP criteria | 3.7 (0.6) | 02–09 | 3.2 (0.3) | 00–06 | −0.78–1.84 | .43 |
| ACB scale | 3.4 (2.2) | 01–09 | 2.9 (1.7) | 00–08 | −0.80–1.79 | .44 |
*Min: minimum; Max: maximum; SD: standard deviation; CI: confidence interval; ICU: intensive care unit; ACB: anticholinergic cognitive burden.
Evidence was obtained that the patients who developed ADRs presented longer hospitalization times (Mean difference=5.21 days, 95%CI=2.32–8.10, p<.001). There was no evidence that the patients who died were exposed to more ADR episodes when compared to those who were discharged to the ward or referred to palliative care. Although the current study did not find evidence of an association between use of PIMs and occurrence of ADRs (Table 4), among the medications classified as PIMs according to the Beers criteria, 3 were responsible for ADRs (lorazepam, quetiapine, and risperidone). In the evaluation by means of the STOPP criteria, 3 situations led to the occurrence of ADRs: duplicated drug class (tramadol and methadone); use of furosemide; and use of 2 or more medications with anticholinergic properties (quetiapine and risperidone).
The estimated prevalence of ADRs in other scientific evidence and according to triggers was between 10.7% and 16.5%, and most of the reactions were classified as probably or possibly caused by a medication.16–18 These data corroborate our research, and it is important to note that it was conducted in a specific context (ICU), where there is a recommendation to use more than one tool for determining causality in intensive care,19 as well as with a population of characteristics that limited the size of our sample (hospitalized older individuals). Thus, these aspects justify the low prevalence of triggers that detected possible ADRs.
Varallo et al.16 showed that some confounders can interfere with the identification of suspected ADRs performed through triggers. These confounding factors are related to the patients' clinical conditions (e.g., renal or hepatic failure and deterioration in the overall health status) and directly imply causal imputation, allowing the ADR to be overestimated or underestimated. Therefore, trigger tools and tools that suggest the causality of ADRs should be used concomitantly to certify the suspicion arising from a medication.16 This is in line with our findings, where most of the triggers identified were associated with the development of diseases in the patients, and the evaluation of the event causality provided guidance for management.
In a number of research studies conducted in hospitals, the use of antihistamines and antiemetics was also among the most reported triggers.17,18,20 Abrupt discontinuation of medication use, excessive sedation (lethargy and falls), and elevated serum creatinine were also highlighted.16,18,20 Pandya et al.17 reported that gastrointestinal disorders (21%) and cardiac disorders (19.3%), followed by skin reactions (17.7%), were the adverse effects detected with the highest prevalence values. In the current study, possible ADRs manifested themselves through gastrointestinal problems and disorders of the nervous and blood/lymphatic system.
The evaluation of ACB in the pharmacotherapy of older people is a recognized practice to help identify ADRs that may compromise the cognitive and physical evolution of these patients and, furthermore, this type of monitoring can contribute to anticipating knowledge about the risk of developing ADRs.8 In the study conducted by Wolters et al.,21 which also evaluated ICU patients, fentanyl was one of the drugs with the highest ACB administered to patients in relation to the number of days surveyed, with morphine and midazolam also standing out. These data are similar to our outcomes, in addition to being associated with the profile of the patients included in the study, who are characterized by the need for analgesia and sedation. However, it should be considered that there is no consensus regarding ACB as a risk factor for ADRs in the ICU context, as the physiological vulnerability of critically ill patients increases their exposure to harmful factors.22 Therefore, theoretically, individuals who present physiological conditions of vulnerability, such as those that alter permeability of the blood–brain barrier, may present a higher risk of developing ADRs in the face of high ACBs and require greater surveillance regarding pharmacotherapy with anticholinergic agents.
In hospital units, including those that provide intensive medical care, benzodiazepines and diuretics are the most common classes of PIMs according to the Beers and STOPP criteria,23 while other realities also point to aspirin, non-steroidal anti-inflammatory drugs, antiplatelet/anticoagulant agents, and proton pump inhibitors (PPIs).24,25 In our research, omeprazole was also one of the most detected PIMs by the instruments. However, not so similarly to the other studies, insulin (rapid-acting) and amiodarone stood out in our screening. Considering that, although they are medications that are inappropriate for use in the older population, sometimes there is no alternative therapy available—as in the cases of insulin and PPIs. Or, even the use of other classes of medications may not be available due to high cost.
Therefore, inclusion of the medication at the lowest therapeutic dose, with adjustments according to kidney and hepatic functions, cautious management, with limited use restricted to the control of acute conditions that require them, are necessary measures for the management of PIM use.26 Prevention strategies through prescription reviews and multidisciplinary discussions also serve as tools, including the possibility of use for the deprescription practice. As a preventive strategy, the risk–benefit of PIM use should be considered and, as soon as the need for its use is extinguished, deprescription should be performed—e.g., in cases of successful extubation and absence of current risks for stress ulcers.
Regarding ADRs, critically ill patients are characterized by using drugs that cause associations involving anticoagulants/antiplatelets, antimicrobials, and anticonvulsants.27,28 It is also evident that medications which act on the nervous system are related to more than 50% of the ADRs in this population segment.28 This is also in line with the particularities of our sample and the suggested pharmacotherapy for the health conditions of this population, considering that patients in the ICU may be anxious, agitated, and in pain and, thus, analgesics and sedatives are widely used.
A multicenter study showed that the mean number of potential ADRs in the ICU was 70.1/1000 drug administrations, dropping to 31.0/1000 administrations when only considering clinically relevant potential ADRs.29 Strategies may include avoiding high-risk drug combinations, increasing patient monitoring when avoidance is not possible, and increasing the clinical relevance of alerts.30 Furthermore, clinical decision-support systems should alert to relevant potential ADRs to avoid alert fatigue for the healthcare team.29,30
Our findings demonstrate an association between the occurrence of ADRs in the ICU and polypharmacy. Polypharmacy, inherent to intensive care, and, in itself, it is presented as a risk condition for the occurrence of adverse events, such as medication errors and ADRs. Furthermore, the occurrence of potential ADRs is high in the ICU setting and, in addition to each patient's individual vulnerability, they may also contribute to the polypharmacy situation and conditions where the clinical outcome of the ADR is desired—e.g., to increase sedation. Thus, it is necessary to direct the multiprofessional team efforts towards actions that guarantee patient safety, with the execution of strategies that ensure rational medication use.
LimitationsThe number of patients included in the study was the main limitation, as it prevented the performance of more robust analyses. During data collection, some factors limited the acquisition of a larger sample size and also developed other weaknesses in our study, such as the following: number of ICU beds (9); inclusion criteria of patients aged ≥60 years old; hospitalization time and, consequently, reduced admission of new potential study participants; amount of data evaluated daily for each patient; and number of triggers in the active search for ADRs.
Despite the limitations presented, this study contributed to describing older patients in intensive care and understanding the need for further studies within the complex, and particular, hospital environment.
Contribution to the scientific literatureThe use of specific tools for identifying the possibility of ADR development in the older population, with directed application to critically ill older patients, helps in the recognition and prevention of important pharmacotherapeutic problems.
FundingThis study was financed in part by the CAPES—Finance Code 001. National Council for Scientific and Technological Development (CNPq, Brazil).
Ethical considerationsThe research project was approved by the Research Ethics Committee of the Faculty of Pharmaceutical Sciences of Ribeirão Preto at the University of São Paulo (CAAE 44000715.6.0000.5403).
Responsibility and assignment of rightsAll authors accept the responsibility defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/). In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation and public communication (by any means or sound, audiovisual or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights will be signed at the time of sending the paper through the online manuscript management system.
CRediT authorship contribution statementFabiana Angelo Marques Carizio: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. Isabella do Vale de Souza: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Alan Maicon de Oliveira: Formal analysis, Writing – original draft, Writing – review & editing. Maria Madalena Corrêa Melo: Formal analysis, Writing – original draft, Writing – review & editing. Maria Olívia Barbosa Zanetti: Writing – original draft, Writing – review & editing. Fabiana Rossi Varallo: Resources, Writing – review & editing. Leonardo Régis Leira-Pereira: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.